By Michael J. Berens and Ken Armstrong
Seattle Times staff reporters
A night-shift nurse slipped into Jeanine Thomas' hospital room and whispered, "I don't know how you're taking this so well. If I were you, I'd be curled up in a ball crying."
The remark mystified Thomas. She'd had ankle surgery, and yes, there had been complications. But she thought she was recovering. Was there something she didn't know?
In November 2000, Thomas, then a 45-year-old antiques dealer, had slipped on ice and shattered her left ankle outside her suburban Chicago home. But days after surgery at her local hospital, the skin surrounding the incisions turned black, and her body swelled. Doctors wanted to amputate, but Thomas, an avid tennis player, refused to let them.
Then, a friend told Thomas about her mother's battle with MRSA, an antibiotic-resistan t germ. Their symptoms matched. Thomas confronted a doctor and learned the truth: She, too, had MRSA. Only now did the nurse's comment make sense.
Thomas asked doctors how many people get MRSA. She was met by silence.
"That's when I knew — a light bulb went on in my head," she says. "They don't want anyone to know about this."
Today, Thomas is exposing MRSA's staggering toll as one of the nation's most influential patient advocates. Because of her persistence, Illinois hospitals now must disclose MRSA infection rates and screen for the germ. She's also pushing for federal legislation that could enhance patient safety in Washington and every other state.
Thomas epitomizes a revolt in health care. A growing number of consumer advocates — many bound by ordeals with MRSA, or methicillin- resistant Staphylococcus aureus — have vowed that if the U.S. Hospital system will not heal itself, they will do it.
Five years ago, not a single state forced hospitals to reveal how many patients contracted infections while under their care. Now 25 states have some form of "report card" disclosure that can make hospitals more accountable.
Washington has a report card; it tracks three kinds of infections — but not MRSA.
MRSA rates in Washington have increased 33-fold in the past decade, a Seattle Times analysis shows. Last year, 4,723 hospital patients were diagnosed with the infection.
With fanfare, the state launched two initiatives last year to combat the epidemic. But in the end, neither made much difference.
In Washington, no patient advocate like Thomas has emerged.
Advocates gain ground
Across the country, consumer advocates have embraced two tools — MRSA screening and hospital report cards — to make hospitals more transparent and aggressive when dealing with infections.
The more popular has been report cards, often a byproduct of patient frustration with hospital secrecy and inadequate infection control.
Chris Cahill, a consumer advocate, worked with legislators to pass a report-card law this year in California.
Before retiring in 2006, Cahill worked for 12 years as a hospital surveyor for the California Department of Health Services. She inspected dozens of hospitals and saw how they could become easy targets for contagion. Some hospitals are "so filthy and dirty it's just incredible," she says.
Many infection-control departments, a cornerstone of patient safety, have so little staff and equipment that it's impossible to track every germ or consistently enforce standards, she says. Even large hospitals often put infection control in the hands of just one or two nurses.
"Many hospitals see infection control as a necessary evil. All they see is the bottom line," Cahill says.
That so many states have recently adopted hospital report cards shows how influential consumer advocates have become. But the hospital industry has often pushed back.
Some hospital officials believe consumers will draw unfair comparisons from report cards, without considering how each hospital has different patient populations. A hospital with a trauma center will have more patients who are vulnerable than one that focuses on elective surgeries.
Lawmakers in some states have passed report cards that provide little information to the public.
In Nevada and Nebraska, hospitals now must report infections to state health officials. But to the public, the numbers remain locked away.
Arkansas encourages hospitals to report infection rates — if they want to. Most don't.
"Not essential right now"
Washington passed its own report-card act in spring 2007. But hospitals have to report only one kind of infection this year: bloodstream maladies in patients who receive a central-line intravenous hookup. The report card will add a second type of infection next year, and a third by 2010. But MRSA is not among them.
Twice before, report-card legislation had died in Washington, after drawing fierce opposition from the hospital industry. The current measure represents a compromise or a first step, said state Rep. Tom Campbell, R-Roy, who sponsored the bill.
In Washington, MRSA has been linked to 1,217 deaths in the past decade, a Seattle Times analysis of hospital records shows. At least 23,707 hospital patients have been diagnosed with MRSA infections.
One Seattle hospital estimates that it costs $20,000 to treat a MRSA infection. Using that figure, MRSA's financial toll in Washington exceeds $474 million.
In the report-card bill, Washington lawmakers had included $240,000 for state health officials to investigate MRSA outbreaks, establish surveillance programs and educate health-care workers and the public about stopping the germ's spread.
The Washington Hospital Association supported the measure, which would have allotted public money to address MRSA.
But Gov. Christine Gregoire stripped the provision out, along with all kinds of other spending items. Her veto notes called the measure "valuable" but "not essential to do right now."
The Illinois fight begins
While hospital report cards have been enacted in much of the country, legislation requiring hospitals to screen patients for MRSA has been difficult to pass.
When Jeanine Thomas contracted MRSA in 2000, no state had even considered such a law. Her success in changing the culture has become a strategic blueprint for consumers in other states, where hospital resistance to mandated screening remains steadfast.
After her ankle healed enough that she could walk, Thomas cobbled together bits and pieces of information about a germ that few seemed to know about.
In 2003, she helped muster support for a bill requiring Illinois hospitals to disclose infection rates. A state senator named Barack Obama co-sponsored the legislation, which passed that year.
The experience inspired Thomas. She began campaigning for a law that would require hospitals to screen patients for MRSA. The screening test, which costs about $20, allows hospitals to identify who has the germ and to isolate them, to protect other patients.
Thomas wrote letters to state legislators and spoke out at health-care meetings. She said doctors undecided about screening had adopted a "compromise of doing nothing."
To combat the medical establishment, she received help from two of the nation's leading infection-control experts. One was Dr. Barry Farr, who was retired from the University of Virginia. Farr had urged hospitals since the 1980s to adopt aggressive screening programs, but he often met with resistance.
The other was Dr. William Jarvis, former acting director of the Centers for Disease Control and Prevention (CDC). Jarvis also supported screening, and sparred with its opponents at infection-control conferences.
"The public is tired of waiting for us to decide this debate and move into action," he says.
By late 2005, Thomas had forged an alliance with Illinois state Sen. Christine Radogno, who agreed to sponsor legislation that would mandate MRSA screening. If passed, the law would be the nation's first.
Thomas later wrote a note about her meeting with Radogno: "She told me tell no one."
They agreed that stealth was necessary because a war was about to begin.
Maryland bill fails
In Maryland, a home contractor from Baltimore had already started a similar war.
Michael Bennett created the Coalition for Patients' Rights after his 88-year-old father, Mark, contracted MRSA at a local hospital. A week after getting MRSA, his father picked up two more antibiotic-resistan t germs. Those infections touched off necrotizing fasciitis — also called flesh-eating disease — and his leg was amputated.
Bennett's father was transferred to a series of rehabilitation centers. There he picked up three more infections, which destroyed his kidneys and poisoned his blood before killing him in June 2004.
While his father was in the hospital, Bennett says, doctors let two months pass before revealing the MRSA diagnosis. During that time, he says, dozens of staffers could have spread the germ. They only occasionally wore gloves or gowns or washed their hands after caring for his father.
In 2005, Bennett helped get legislation introduced to mandate MRSA screening in Maryland.
"Hospital infections have been killing too many for far too long," he says.
But the bill received what Bennett calls a "barrage of criticism" from hospital-industry officials, and it went down to defeat. Bennett tried again in 2006 and 2007, but with the same result.
The first breakthrough
When the Illinois MRSA legislation was introduced in January 2006, a firestorm erupted.
National medical groups attacked the bill, challenging screening's benefits and costs while touting existing infection-control measures, such as hand hygiene. The bill died in a House committee.
But Thomas tried again.
The first public hearing was held in February 2007 in downtown Chicago, during a fierce snowstorm. Many opponents showed up; Thomas was the lone supporter.
The Illinois Hospital Association originally opposed Thomas, fearing her proposal would force hospitals to test every patient. Later, when assured the scope was limited to critically ill patients and others at high risk of contracting MRSA, the association supported the measure.
The association had conducted research of patient-discharge data — the same kind of analysis The Seattle Times did in Washington — and was stunned at how many MRSA cases it found, Thomas says.
In May 2007, the bill passed the Illinois Legislature. The House vote was 106-0.
Thomas immediately called Farr: "He was overcome. He said he had waited so long for this."
But in August, an aide to Gov. Rod Blagojevich called and told Thomas the governor intended to veto the bill. His office issued a news release saying as much. Thomas, crying, began working the phones, rallying legislators to flood the governor with calls.
A few hours later, the governor changed his mind and signed the bill.
Three other states — Pennsylvania, New Jersey and California — have since passed similar screening laws.
"Impractical or extreme"
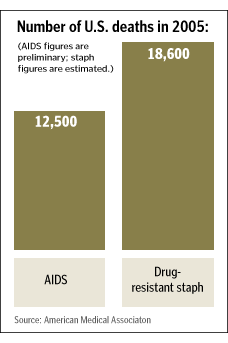
Washington, like the rest of the country, was rattled last October by the highly publicized announcement of the federal Centers for Disease Control and Prevention that MRSA now kills more people than AIDS.
Some schools closed temporarily over a single MRSA infection, or canceled football games so locker rooms could be disinfected.
In November, Gregoire wrote to the state Health Department, saying: "We need to do more." She ordered the agency to collect MRSA test results from medical laboratories statewide, and to create a panel of experts to recommend ways to curb MRSA's spread.
"Gregoire takes on superbug," a Seattle Times headline said.
But a year later, these two unfunded initiatives have had little effect.
The reporting of test results was voluntary, so some labs did not submit them. In other cases, the information was so sketchy that state officials couldn't tell where people caught the germ.
"We didn't find a whole lot of meaningful data," says Judith May, the Health Department's acting director of epidemiology.
As for the expert panel, it was stacked with hospital representatives, without a single consumer advocate. In January, the 18-member panel published its MRSA report — an 82-page rehash of existing medical literature.
The panel opposed screening all patients for MRSA, calling it "impractical or extreme ... with little added value."
Instead, it recommended that hospitals use infection-control guidelines from the CDC. But those guidelines have been widely criticized by congressional investigators, who call them confusing and conflicting.
In Washington, most community hospitals make these guidelines the core of their infection-control programs. None tests every patient for MRSA.
Last month, a representative of the Illinois Hospital Association met with a few dozen Washington hospital officials and touted the benefits of widespread MRSA screening.
For the first time, Illinois is getting a true picture of MRSA's toll, the representative told them.
Last year, the Illinois hospital group found approximately 11,300 MRSA cases. But this year, with screening in full force, the group estimates the number could be close to 30,000.
The fight continues
Today, Jeanine Thomas runs the MRSA Survivors Network.
She's haunted by memories of her time in the hospital, when she rolled up and down the corridors in a wheelchair, or grabbed coffee in the cafeteria, or used the drinking fountains.
"For two months I didn't know I had this infectious germ. The hospital let me go wherever I wanted.
"How many people did I infect?"
These days, she is campaigning for a federal law to mandate hospital MRSA screening in every state.
She criticizes Washington's refusal to embrace widespread screening, saying many of its hospitals are endangering patients.
Her Web page links to survivor stories, from a mother who lost her 7-week-old daughter to MRSA to a 58-year-old man who lost much of his left leg.
Thomas points to these survivors when confronting critics of MRSA screening.
"I'm trying to save lives," she tells them. "What are you trying to save?"
Michael J. Berens: mberens@seattletime s.com or 206-464-2288; Ken Armstrong: karmstrong@seattlet imes.com or 206-464-3730. Reporter Justin Mayo and researchers David Turim and Gene Balk contributed.
Copyright © 2008 The Seattle Times Company
Tuesday, November 25, 2008
Bet the Hospital & Doctors are Doing Fine
 Staph infections have ruined the life of Brook Park man
Staph infections have ruined the life of Brook Park manby Harlan Spector/Plain Dealer Reporter
Saturday November 22, 2008, 7:26 PM
Photo Marvin Fong/The Plain Dealer
Roger Chorich has battled staph infections for years, culminating in amputation of both his legs.
BROOK PARK -- Roger Chorich's days are a blur of TV watching and bumping around the house in a wheelchair. He pops painkillers and antidepressants, and obsesses about bill collectors.
He will never know for sure how he got to such a dark place. Wracked with intractable infections since double knee replacements four years ago, he finally had to have his left leg amputated in August 2007. Then last month doctors took the right leg.
How was infectious staph able to burrow deep into his joints and render him a double amputee?
Was the hospital less than sterile?
Who's at fault?
Anyone?
The questions become more searing with time, as he and his wife, Anita, fall further behind on bills. He stares out the windows of their small brick ranch, at the yard he once planted with impatiens and marigolds, waiting for a certified letter announcing foreclosure proceedings.
He's 58 and lives on a $1,600-a-month pension from the U.S. Postal Service. They're in bankruptcy. His medical needs are too great for Anita to take on full-time work. She's always hauling him to the doctor, sliding him into the Ford Taurus with a board placed under his torso.
"We're probably going to lose the house," Chorich said, his voice tight with tension. "I'm just angry with the whole world, I guess. I just don't know where to go."
His long battle with staph has been chronicled twice before in Plain Dealer stories about hospital infections. Antibiotic-resistant staph, the same type that has sidelined a growing roster of pro football players, spread throughout Chorich's body after the knee replacements. He turned to Dr. John Sontich, an orthopedic surgeon at MetroHealth Medical Center, who again and again has cut into Chorich's ravaged knee implants and infected bone.
The final blow to Chorich's right knee was an uncontrollable yeast infection caused by years of antibiotic use. It was his third knee implant on that side, and it harbored resistant staph bacteria as well. Sontich had no choice but to amputate one-third up the femur.
Sontich said he's hoping that without artificial joints to hide in, the staph infections are gone for good.
No more antibiotics.
No more painkillers.
"He's going to be able to walk with crutches or maybe a cane," the doctor said.
Chorich has been down this road before, thinking the staph was gone, only to feel piercing pain return.
"It's like cancer," said Anita, who was treated for breast cancer six years ago. "I'm already afraid it's [staph] going to come back."
Chorich is hoping to eventually walk on prosthetics. The left one is now gathering dust in a corner in the bedroom, while he awaits a prosthetic for the right. He hopes to work again, and chip away at a debt pile of about $150,000. Anita has a temporary job running the kiddie train at Great Northern Mall three days a week. She applied to be an usher or ticket taker at Progressive Field in the spring.
"Maybe I could be doing something inside," Chorich said. "I've never done it. I've been a laborer. If someone would train me, I'd do it."
People have helped out here and there.
Leona Osrin of Beachwood wrote to Chorich and sent a check after reading about his ordeal. She recently sent him another $100.
"To have to live this way is absolutely horrific," Osrin said in an interview. "My few dollars isn't going to change his life, but it shows somebody cares about him."
Anita has hope. She thinks they will be able to renegotiate terms of a $90,000 home equity loan. "I don't worry as much as him. I always hope things are going to get better," she said.
Chorich bristled. "If we don't pay for the house, they're going to come after you," he snapped at her. "We're in trouble."
The combination of pills, depression, immobility and idle time has taken a toll.
She grabbed her packed lunch from the kitchen. "He needs to talk to somebody," she confided as she passed. Then she kissed him goodbye and left for the mall.
Tuesday, November 4, 2008
Meet Logan, The Sky Angel Cowboy - CBN News
If you have lost someone that you care about, if you have endured a long and painful illness, if you find yourself feeling isolated and alone...angry at God and the world. You will be inspired by this young boy.
Thursday, October 2, 2008
Don't Mess with Maria!
Schwarzenegger creates hospital privacy oversight office
The move comes months after his wife, Maria Shriver, and other celebrities had their medical records peeked at by employees at UCLA.
By Patrick Mcgreevy October 01, 2008
SACRAMENTO – Gov. Arnold Schwarzenegger took action today to enable the state to impose stiff fines on hospital employees who snoop in their patients’ files, months after California First Lady Maria Shriver was one of several celebrities whose privacy was invaded at UCLA Medical Center.
The governor approved the creation of a new state Office of Health Information Integrity with power to review security plans and violations and assess fines of up to $250,000 against violators of patient privacy.
The governor’s decision follows a series of reports by The Times during the last year that at least 127 employees at UCLA peeked at the confidential medical records of celebrities including Britney Spears, Farrah Fawcett and Shriver.
“Repeated violations of patient confidentiality are potentially harmful to Californians, which is why financial penalties are needed to ensure employees and facilities do not breach confidential medical information,” Schwarzenegger said in a statement after signing AB 211, by Assemblyman Dave Jones (D-Sacramento), which creates the new oversight office and fines on individuals.
He also signed a companion bill that allows fines of up to $250,000 against hospitals and health clinics for such breaches and increases the maximum fine for serious medical errors from the current $50,000 to $125,000. SB 541 was written by Sen. Elaine Alquist (D-Santa Clara).
Schwarzenegger was outraged when his wife’s records were breached by an employee, saying no one should have that happen.
“Californians seeking care at a hospital or health facility should never have to worry that their private medical information will be shared,” the governor said today.
patrick.mcgreevy@latimes.com
The move comes months after his wife, Maria Shriver, and other celebrities had their medical records peeked at by employees at UCLA.
By Patrick Mcgreevy October 01, 2008
SACRAMENTO – Gov. Arnold Schwarzenegger took action today to enable the state to impose stiff fines on hospital employees who snoop in their patients’ files, months after California First Lady Maria Shriver was one of several celebrities whose privacy was invaded at UCLA Medical Center.
The governor approved the creation of a new state Office of Health Information Integrity with power to review security plans and violations and assess fines of up to $250,000 against violators of patient privacy.
The governor’s decision follows a series of reports by The Times during the last year that at least 127 employees at UCLA peeked at the confidential medical records of celebrities including Britney Spears, Farrah Fawcett and Shriver.
“Repeated violations of patient confidentiality are potentially harmful to Californians, which is why financial penalties are needed to ensure employees and facilities do not breach confidential medical information,” Schwarzenegger said in a statement after signing AB 211, by Assemblyman Dave Jones (D-Sacramento), which creates the new oversight office and fines on individuals.
He also signed a companion bill that allows fines of up to $250,000 against hospitals and health clinics for such breaches and increases the maximum fine for serious medical errors from the current $50,000 to $125,000. SB 541 was written by Sen. Elaine Alquist (D-Santa Clara).
Schwarzenegger was outraged when his wife’s records were breached by an employee, saying no one should have that happen.
“Californians seeking care at a hospital or health facility should never have to worry that their private medical information will be shared,” the governor said today.
patrick.mcgreevy@latimes.com
Thursday, September 18, 2008
Unsanitary Dialysis Center Shuts Down Over Hepatitis
By ANEMONA HARTOCOLLIS
Published: September 16, 2008
A Manhattan dialysis center closed down after State Health Department inspectors found blood on chairs and machines and discovered that at least one patient had contracted hepatitis C because of the unsanitary conditions.
More than 600 other patients treated at the center, going back nearly five years, were urged to get hepatitis and H.I.V. tests.
Claudia Hutton, a spokeswoman for the Health Department, said on Tuesday that it inspected the clinic, the Life Care Dialysis Center, at 221 West 61st Street, for a week in mid-August and found that employees had failed to wash their hands properly, disinfect equipment or change gloves between patients. Inspectors also found blood on treatment chairs.
“It was repulsive,” Ms. Hutton said. “The treatment chairs that they gave people to relax in had someone else’s dried blood on them.”
Ms. Hutton said that the clinic was ordered to begin sending its patients to other clinics immediately while the Health Department began testing patients for signs of infection.
She said that when one patient was found to have been infected by hepatitis C, a liver disease, because of contaminated equipment, the clinic shut down voluntarily.
Richard F. Daines, the state’s health commissioner, sent letters on Monday to 657 patients of the clinic going back to January 2004, the last time infection-control violations had been found, urging them to be tested for possible exposure to hepatitis C, hepatitis B and H.I.V. The clinic, which had 171 patients at the time of the inspection, agreed to pay for the testing, even if it is done by private doctors, officials said.
Ms. Hutton said there was no evidence that other patients had been infected.
Dr. Walter Wasser, the clinic’s operator and medical director, was fined $300,000 and surrendered his operating certificate, Ms. Hutton said. He could face the loss of his medical license after an investigation by the State Office of Professional Medical Conduct, Ms. Hutton said. She declined to say whether such an investigation had begun.
Dr. Wasser did not return a call for comment left with his answering service.
Ms. Hutton said that the Health Department visited the clinic in August to follow up on previous violations, not because of any specific new complaints from patients. She said the department tried to inspect clinics once a year, but was sometimes not able to do so that often because of manpower shortages and the volume of complaints.
She said the department was particularly concerned about dialysis clinics because their patients have compromised immune systems that make them vulnerable.
http://www.nytimes.com/2008/09/17/nyregion/17dialysis.html?_r=2&ref=nyregion&oref=slogin&oref=slogin
Published: September 16, 2008
A Manhattan dialysis center closed down after State Health Department inspectors found blood on chairs and machines and discovered that at least one patient had contracted hepatitis C because of the unsanitary conditions.
More than 600 other patients treated at the center, going back nearly five years, were urged to get hepatitis and H.I.V. tests.
Claudia Hutton, a spokeswoman for the Health Department, said on Tuesday that it inspected the clinic, the Life Care Dialysis Center, at 221 West 61st Street, for a week in mid-August and found that employees had failed to wash their hands properly, disinfect equipment or change gloves between patients. Inspectors also found blood on treatment chairs.
“It was repulsive,” Ms. Hutton said. “The treatment chairs that they gave people to relax in had someone else’s dried blood on them.”
Ms. Hutton said that the clinic was ordered to begin sending its patients to other clinics immediately while the Health Department began testing patients for signs of infection.
She said that when one patient was found to have been infected by hepatitis C, a liver disease, because of contaminated equipment, the clinic shut down voluntarily.
Richard F. Daines, the state’s health commissioner, sent letters on Monday to 657 patients of the clinic going back to January 2004, the last time infection-control violations had been found, urging them to be tested for possible exposure to hepatitis C, hepatitis B and H.I.V. The clinic, which had 171 patients at the time of the inspection, agreed to pay for the testing, even if it is done by private doctors, officials said.
Ms. Hutton said there was no evidence that other patients had been infected.
Dr. Walter Wasser, the clinic’s operator and medical director, was fined $300,000 and surrendered his operating certificate, Ms. Hutton said. He could face the loss of his medical license after an investigation by the State Office of Professional Medical Conduct, Ms. Hutton said. She declined to say whether such an investigation had begun.
Dr. Wasser did not return a call for comment left with his answering service.
Ms. Hutton said that the Health Department visited the clinic in August to follow up on previous violations, not because of any specific new complaints from patients. She said the department tried to inspect clinics once a year, but was sometimes not able to do so that often because of manpower shortages and the volume of complaints.
She said the department was particularly concerned about dialysis clinics because their patients have compromised immune systems that make them vulnerable.
http://www.nytimes.com/2008/09/17/nyregion/17dialysis.html?_r=2&ref=nyregion&oref=slogin&oref=slogin
Wednesday, September 10, 2008
Special Dyes and Lighting Kill MRSA, Research Shows
But new technologies don't replace basic infection control procedures, expert says
By Steven Reinberg, HealthDay Reporter
WEDNESDAY, Sept. 10 (HealthDay News) -- A new kind of paint that releases titanium dioxide when exposed to fluorescent light and a green dye for wounds that gives off toxic molecules when activated by near-infrared light could both kill the deadly superbug known as MRSA, two new studies claim.
MRSA stands for methicillin-resistant Staphylococcus aureus. It's a strain of staph that's resistant to many antibiotics commonly used to treat it, and it can be fatal. Both reports were presented Tuesday at the Society for General Microbiology Autumn meeting at Trinity College in Dublin.
In the first study, British researcher Lucia Caballero, from Manchester Metropolitan University, found that paint that contained particles of titanium dioxide killed bacteria when it absorbed ultraviolet light.
"If this turns out, the impact is sure to be positive in the area of health," Caballero said.
The same reaction occurs when paints containing titanium dioxide are exposed to infrared light. The researchers found that the paint containing titanium dioxide successfully killed bacteria when the concentration of these nanoparticles was stronger than the normal paint. In fact, they found that all E. coli were killed with fluorescent lights.
"There are many circumstances where it is necessary or desirable to remove or to kill microorganisms found in a biological host or on surfaces," Caballero said. "Maintenance of hygienic standards is essential in hospitals, pharma and the food industry. Surface hygiene could be improved by the action of fluorescent light on catalytic surfaces, such as paints containing nanotitanium, for retarding contamination and saving on cost of cleaning maintenance."
In the second report, Dr. Ghada Omar, from University College London, found that 99 percent of the MRSA bacteria in infected wounds could be killed using a green dye that gives off toxic molecules when activated by infrared light.
"The chemicals produced when the dye is activated harm the bacteria in such a wide variety of ways that it is unlikely bacteria could ever develop resistance to the treatment," Omar said in a statement. "This makes it ideal, and possibly the only option, for treating infections with multiple drug-resistant bacteria, including MRSA."
Infected wounds are a major problem for thousands of hospital patients. Up to 9 percent of hospital-acquired infections occur during surgery and contribute to 77 percent of deaths from operations. These infections increase the length of time patients remain in hospital and increase costs, Omar noted.
Dr. Pascal James Imperato, Dean and Distinguished Service Professor of the Graduate Program in Public Health at SUNY Downstate Medical Center in Brooklyn, said a lot more work needs to be done before these technologies become practical.
"It's very much in the experimental stage at this point," Imperato said. "It's an interesting new development, but one is going to have to see many more studies to determine whether or not this is really going to work."
Dr. Marc Siegel, an associate professor of medicine at New York University School of Medicine, thinks that basic cleanliness can do more to remove the threat of hospital infections than these new technologies.
"This technology is very promising, but it gets us away from the real issue, which is that we have a problem with cleanliness and sterility in hospitals," Siegel said. "The real issue is that doctors don't change their coats, change their gloves enough. We are not using hand wipes."
Siegel noted that soap and water, when used properly, gets rid of most of this bacteria.
"MRSA has been around for 20 years and is a symptom of our inability to properly clean and sterilize facilities, while, at the same time, we are overusing antibiotics," Siegel said.
For more on MRSA, visit the U.S. Centers for Disease Control and Prevention.
SOURCES: Lucia Caballero, Manchester Metropolitan University, U.K.; Marc Siegel, M.D., associate professor, medicine, New York University School of Medicine, New York City, and author, Bird Flu: Everything You Need to Know About the Next Pandemic; Pascal James Imperato, M.D., M.P.H., dean and distinguished service professor, Graduate Program in Public Health, SUNY Downstate Medical Center, Brooklyn, New York; Sept, 9, 2008, presentations, Society for General Microbiology Autumn meeting, Trinity College, Dublin
By Steven Reinberg, HealthDay Reporter
WEDNESDAY, Sept. 10 (HealthDay News) -- A new kind of paint that releases titanium dioxide when exposed to fluorescent light and a green dye for wounds that gives off toxic molecules when activated by near-infrared light could both kill the deadly superbug known as MRSA, two new studies claim.
MRSA stands for methicillin-resistant Staphylococcus aureus. It's a strain of staph that's resistant to many antibiotics commonly used to treat it, and it can be fatal. Both reports were presented Tuesday at the Society for General Microbiology Autumn meeting at Trinity College in Dublin.
In the first study, British researcher Lucia Caballero, from Manchester Metropolitan University, found that paint that contained particles of titanium dioxide killed bacteria when it absorbed ultraviolet light.
"If this turns out, the impact is sure to be positive in the area of health," Caballero said.
The same reaction occurs when paints containing titanium dioxide are exposed to infrared light. The researchers found that the paint containing titanium dioxide successfully killed bacteria when the concentration of these nanoparticles was stronger than the normal paint. In fact, they found that all E. coli were killed with fluorescent lights.
"There are many circumstances where it is necessary or desirable to remove or to kill microorganisms found in a biological host or on surfaces," Caballero said. "Maintenance of hygienic standards is essential in hospitals, pharma and the food industry. Surface hygiene could be improved by the action of fluorescent light on catalytic surfaces, such as paints containing nanotitanium, for retarding contamination and saving on cost of cleaning maintenance."
In the second report, Dr. Ghada Omar, from University College London, found that 99 percent of the MRSA bacteria in infected wounds could be killed using a green dye that gives off toxic molecules when activated by infrared light.
"The chemicals produced when the dye is activated harm the bacteria in such a wide variety of ways that it is unlikely bacteria could ever develop resistance to the treatment," Omar said in a statement. "This makes it ideal, and possibly the only option, for treating infections with multiple drug-resistant bacteria, including MRSA."
Infected wounds are a major problem for thousands of hospital patients. Up to 9 percent of hospital-acquired infections occur during surgery and contribute to 77 percent of deaths from operations. These infections increase the length of time patients remain in hospital and increase costs, Omar noted.
Dr. Pascal James Imperato, Dean and Distinguished Service Professor of the Graduate Program in Public Health at SUNY Downstate Medical Center in Brooklyn, said a lot more work needs to be done before these technologies become practical.
"It's very much in the experimental stage at this point," Imperato said. "It's an interesting new development, but one is going to have to see many more studies to determine whether or not this is really going to work."
Dr. Marc Siegel, an associate professor of medicine at New York University School of Medicine, thinks that basic cleanliness can do more to remove the threat of hospital infections than these new technologies.
"This technology is very promising, but it gets us away from the real issue, which is that we have a problem with cleanliness and sterility in hospitals," Siegel said. "The real issue is that doctors don't change their coats, change their gloves enough. We are not using hand wipes."
Siegel noted that soap and water, when used properly, gets rid of most of this bacteria.
"MRSA has been around for 20 years and is a symptom of our inability to properly clean and sterilize facilities, while, at the same time, we are overusing antibiotics," Siegel said.
For more on MRSA, visit the U.S. Centers for Disease Control and Prevention.
SOURCES: Lucia Caballero, Manchester Metropolitan University, U.K.; Marc Siegel, M.D., associate professor, medicine, New York University School of Medicine, New York City, and author, Bird Flu: Everything You Need to Know About the Next Pandemic; Pascal James Imperato, M.D., M.P.H., dean and distinguished service professor, Graduate Program in Public Health, SUNY Downstate Medical Center, Brooklyn, New York; Sept, 9, 2008, presentations, Society for General Microbiology Autumn meeting, Trinity College, Dublin
Monday, September 8, 2008
A Real Look at the Joint Commission
Value of hospital accreditations under review
By YAMIL BERARD yberard@star-telegram.com
Christine Cahill, a government inspector, walked in to the operating room of a Los Angeles county hospital and found a technician cleaning a surgical instrument. He told her that he had just washed it, but she noticed no water in the sink, so she questioned how he had cleaned it, and he said he had used a cleaner that was in a bottle on the shelf.
"Give me a Q-Tip," she said. She shoved it into the hollow bore of the instrument.
"Out came this crud," she recalled. It was dried-up fragments of bone and blood.
At one out of every three hospitals Cahill surveyed for the federal government in California from 2004-06, she said she found egregious deficiencies that put patients’ lives at risk. Yet these same hospitals, within a year before her review, had received passing grades from the Joint Commission, America’s top healthcare evaluator.
It gave its most prestigious honor — its trademark Gold Seal of Approval — to Martin Luther King Jr./Drew Medical Center, where Cahill found the filthy surgical instrument.
And the commission also awarded that top honor, symbolizing that a hospital has met the most rigorous standards for patient care and safety, to John Peter Smith Hospital in Fort Worth in spring 2006 — the year before an independent consultant documented pervasive problems that put patients at risk.
Now, the Joint Commission itself is under review. For the first time in three decades, Congress is requiring the commission to reapply for authority to certify that hospitals meet federal standards. The commission has a virtual monopoly on hospital accreditation; 88 percent of the nation’s hospitals now choose it over a state agency.
The commission isn’t flinching over the new requirement. It posted a statement on its Web site saying it "is confident that it will receive deeming authority."
But Congress’ rare move opens up the application process to other organizations — including one in Houston that is drawing the interest of a number of Texas hospitals. Some member hospitals are griping that commission reviews often depart from Medicare standards, creating more compliance work.
For critics who characterize the relationship between the commission and client hospitals as too cozy, there couldn’t be a better time to shake up the status quo.
The situation resembles a "country-club-like setting," said Dr. Sidney Wolfe, director of Public Citizen’s Health Research Group in Washington, D.C., a consumers group. "What’s the point of having a regulator that’s a cheerleader over the institution they are supposed to be regulating?"
Scandals also fired up congressional debate last year involving the value of the commission’s accreditation. At issue were cases of accredited hospitals where patients got grossly inadequate treatment. One was a 14-bed West Texas facility where a patient died and federal officials reported that numerous staff members did not have adequate training for the medical procedures they were performing.
As a response to critics, the commission has been working in recent years to beef up its reviews. So-called unannounced visits — in which hospitals are given 48 hours’ warning — began a few years ago. Surveyors now track patients in the operating room and question doctors about diagnoses. What’s more, the agency responds not only to patient concerns, but to news reports of problems, as they did with JPS.
Commission surveyors arrived unannounced at JPS in June following Star-Telegram reports describing the JPS trauma center as a war zone, operating rooms as chaotic, instruments broken, rooms dirty, linens threadbare. Patients were put at risk when doctors couldn’t locate lab results or get crucial reports from specialists.
"We take all complaints seriously," commission spokeswoman Elizabeth Zhani said.
The inspectors cited a list of deficiencies, including intrusions into patient privacy, use of outdated drugs, filth and a system that could not keep track of narcotics, hospital officials said.
Such disturbing findings don’t mean the accreditation process is flawed, Zhani said.
"Accreditation is just not a [survey] visit," she said. "A lot of people tend to focus on that. We don’t know everything else that goes on."
"We cannot be there 365 days of the year," Zhani said.
But critics say the commission will be hard-pressed to identify a hazard so serious that it will shut down a hospital. It didn’t pull the accreditation from Martin Luther King Jr./Drew Medical Center until a year after Cahill conducted her survey — and the news media highlighted patient deaths tied to staff errors.
The Joint Commission process "in itself is, to say the least, superficial," Cahill said. "It doesn’t tell you what the hospital is doing."
Survey problems
The Joint Commission’s plan of attack has always been to "peer-review" or educate hospitals about quality standards, not regulate them, Zhani said.
"Organizations who contract with the Joint Commission are voluntarily saying: 'We want you to come in and survey us and inspect us to see if we’re following your standards for patient care,’ " Zhani said. "They are making a commitment that they want to focus on quality improvement."
But even supporters say the survey process has weaknesses.
Hospitals, no matter the size, are surveyed at least every three years. And the inspectors — usually independent contractors, not commission employees — spend about three days, often in back-to-back interviews with hospital administrators. Some say that doesn’t allow sufficient time for investigation.
At times, medical records are reviewed to make sure the proper documentation is there, not that patients received the proper medication, critics say. The focus of the surveys is on process rather than outcome, said Emily Moreno, a San Antonio registered nurse, now retired, who spent 30 years doing quality assurance at hospitals.
Even at that, though, a surveyor might have found what the JPS consultant did — that up to 20 percent of all medical records were missing.
It also isn’t clear to what degree hospital officials direct surveyors to departments or whether surveyors can — or have time to — wander around and just freely observe. That could explain why troubling problems were apparently overlooked at JPS.
Because the Joint Commission doesn’t make its reports available to the public, patients can’t be assured that surveyors visited some of the less conspicuous areas of the hospital, such as the unit that houses Tarrant County Jail inmates.
An independent consultant in 2007 noted a "dungeon like feel" to the unit and said that infectious patients were housed with noninfectious ones, with the potential of exposing patients, staff and sheriff’s deputies to such diseases as tuberculosis. Staff members told the consultant that the toilets were usually dirty and smelly, garbage cans regularly overflowed. Furniture was broken, and workspaces put doctors and nurses at risk of attack.
The Star-Telegram was not able to interview commission inspectors, despite repeated attempts.
When the survey turns up deficiencies, the commission works with hospitals to help them meet standards. And the commission continues to unveil better methods of identifying patient safety risks. One is a new practice in which surveyors "trace" or follow patients in surgery and other critical services in order to look for vulnerabilities in a hospital’s management of their care.
But critics say that the Joint Commission has other interests that may trump patient safety and lead the commission to stymie efforts that would strengthen compliance and public awareness of hospital problems.
For one, it relies on hospitals for the lion’s share of its $108 million in revenue. Hospitals pay tens of thousands of dollars for their survey evaluations. What’s more, the commission sells its consulting services to help hospitals meet accreditation standards. That boosts sales and creates the potential for conflicts of interest.
"It’s basically run by the [hospital] industry," said Julia Greene, a healthcare professional in Chicago with the Service Employees International Union, which has 2 million members, including the nation’s largest healthcare union.
Among those seeking change is the American Nurses Association, which blames the U.S. Department of Health and Human Services for failing to adequately supervise the commission’s role in the accreditation of hospitals.
Pressure is also coming from some groups concerned about rising healthcare costs. Business groups and insurers want to know if they are getting effective care for their money.
The Office of the Inspector General of DHHS is also expected to add fuel to the fire to revamp the commission. By winter, it is expected to release an examination of the commission’s accreditation process. The inspector general last reviewed the nonprofit in a scathing 1999 report that said its inspections were superficial and left little time for real scrutiny of a hospital’s errant practitioners — the ones most apt to do harm to patients.
Checks and balances
The government does provide some checks and balances to the commission in the form of federal validation surveys, such as Cahill’s at Martin Luther King Jr./Drew, which closed in August 2007 because of substandard conditions.
The surveys are conducted to validate the processes of the commission — "to make sure they’re doing what they’re supposed to be doing," Cahill said.
At most, only 5 percent of the nation’s hospitals undergo the federal scrutiny each year, she said.
But they always involve a team of specialists who spend a longer period studying a hospital, relying on a set of 19 health quality "conditions" required under Medicare regulations. For the MLK Jr./Drew Medical Center survey, Cahill was teamed with pharmacists, nutritionists, nurses and physicians. The surveys can last up to five days, and surveyors can visit the hospital four or five times to check to see that the hospital has corrected problems.
"When [government inspectors] do a validation survey based on the federal regulations, they are very, very thorough and the survey process is a much better process at finding problems than the Joint Commission would ever be," Cahill said.
While inspectors will suggest ways to improve, the survey is intended to root out bad practices, Cahill said.
If a medical record is reviewed, for example, government inspectors observe the patient’s care to see if the practice matches the hospital’s policy and federal standards, she said.
"We probe it further until we are convinced that it’s not an issue or it is an issue," she said.
That’s how inspectors found the case of a meningitis patient who had mistakenly received a potent anticancer drug for four days at the Los Angeles hospital.
Patients may also get some protection from state enforcement agencies. In June, Texas health inspectors were quick to arrive at JPS after news reports drew the commission back for a closer look. State inspectors are closer to the action and can add more layers of protection, the DHHS inspector general reported in 2000.
And next year, there may be a new face or two arriving to accredit hospitals in Texas. A Houston-based accreditation company — DNV Healthcare — is drawing the interest of a number of hospitals who say the commission’s standards deviate too far from Medicare rules.
The commission may have to reckon with the idea that, over time, it could lose its virtual monopoly on accreditation.
That’s all right with the Texas Hospital Association.
Competition "is a good thing . . .," says Starr West, senior director of policy analysis for the association.
"This sort of levels the playing field. We’ve been very supportive of having competition . . . it kind of keeps everybody in line."
Changes in hospitals sought Consumer advocates and other critics are pressing the Joint Commission to strengthen public accountability of hospitals. These are some of the changes being sought.
1 Disclose more information, including life-threatening medical errors and hospital deficiencies.
A commission manual states that hospitals are "encouraged" to report sentinel events, including patient suicide, abduction of newborns, foreign objects left in patients’ bodies during surgery and other major events. But hospitals are not required to do so. When a hospital does report, the commission usually does not identify it to the public. A national snapshot of reported sentinel events on the Joint Commission Web site shows just a map of the number of incidents by state.
Releasing the names of hospitals where sentinel events occurred would be a small step in protecting the public, said Lisa McGiffert, senior policy analyst at Consumers Union in Austin, which publishes Consumer Reports. But even that wouldn’t provide the public with information it needs, she said. For example, the Centers for Disease Control and Prevention estimates that almost 100,000 people die of hospital-acquired infections each year, yet in the past 13 years, the Joint Commission has classified only 105 cases as sentinel events, she said.
"If these sentinel events weren’t so misleading to the public, it’d be laughable," McGiffert said.
"The public has this belief that someone else is watching and making sure that hospitals are safe, and they are mistaken. Nobody is watching."
2 Make survey reports available to the public.
On its Web site, the commission publishes "Quality Reports" that compare a hospital’s performance in a handful of areas to that of other hospitals. For example, John Peter Smith Hospital’s report shows that in 2007 it rated below most accredited hospitals in the country when it comes to heart attack care. ( www.qualitycheck.org/qualityreport.aspx?hcoid=9048#)
But it’s left up to hospitals to decide whether to disclose deficiencies noted by surveyors. After commission inspectors paid a surprise visit to JPS this summer, the hospital made public some findings but did not release the report and specific deficiencies.
"If the organization wants to release the report, they can release it," Joint Commission spokeswoman Elizabeth Zhani said.
3 Pay more heed to patient complaints.
Emily Moreno of San Antonio, who focused on hospital quality during her 30 years as a registered nurse, filed a complaint with the commission after she developed an infection at the site of a breast biopsy at an Oklahoma City hospital. The commission had accredited the hospital seven months earlier.
It took no action on her complaint, she said. It only told her, in response, that it gives "serious consideration" to all complaints.
Susan Sheridan, an Idaho mother of a child with cerebral palsy, did get the commission’s attention. Her son, now 13, suffered brain damage because of the failure of hospitals to test newborns for jaundice. She and other mothers of children with cerebral palsy co-founded Parents of Infants of Children with Kernicterus. Thanks to her advocacy, hospitals now test for excessive bilirubin levels in newborns.
4 Place more patient representatives on the commission board.
Sheridan would also like to see the commission open up more hospital data to the public, but doesn’t believe that will happen soon.
"Their very structure stifles that," she said. Most of its board members are doctors or health practitioners.
"What ties their hands basically is that its members and their clients are hospitals," she said.
By YAMIL BERARD yberard@star-telegram.com
Christine Cahill, a government inspector, walked in to the operating room of a Los Angeles county hospital and found a technician cleaning a surgical instrument. He told her that he had just washed it, but she noticed no water in the sink, so she questioned how he had cleaned it, and he said he had used a cleaner that was in a bottle on the shelf.
"Give me a Q-Tip," she said. She shoved it into the hollow bore of the instrument.
"Out came this crud," she recalled. It was dried-up fragments of bone and blood.
At one out of every three hospitals Cahill surveyed for the federal government in California from 2004-06, she said she found egregious deficiencies that put patients’ lives at risk. Yet these same hospitals, within a year before her review, had received passing grades from the Joint Commission, America’s top healthcare evaluator.
It gave its most prestigious honor — its trademark Gold Seal of Approval — to Martin Luther King Jr./Drew Medical Center, where Cahill found the filthy surgical instrument.
And the commission also awarded that top honor, symbolizing that a hospital has met the most rigorous standards for patient care and safety, to John Peter Smith Hospital in Fort Worth in spring 2006 — the year before an independent consultant documented pervasive problems that put patients at risk.
Now, the Joint Commission itself is under review. For the first time in three decades, Congress is requiring the commission to reapply for authority to certify that hospitals meet federal standards. The commission has a virtual monopoly on hospital accreditation; 88 percent of the nation’s hospitals now choose it over a state agency.
The commission isn’t flinching over the new requirement. It posted a statement on its Web site saying it "is confident that it will receive deeming authority."
But Congress’ rare move opens up the application process to other organizations — including one in Houston that is drawing the interest of a number of Texas hospitals. Some member hospitals are griping that commission reviews often depart from Medicare standards, creating more compliance work.
For critics who characterize the relationship between the commission and client hospitals as too cozy, there couldn’t be a better time to shake up the status quo.
The situation resembles a "country-club-like setting," said Dr. Sidney Wolfe, director of Public Citizen’s Health Research Group in Washington, D.C., a consumers group. "What’s the point of having a regulator that’s a cheerleader over the institution they are supposed to be regulating?"
Scandals also fired up congressional debate last year involving the value of the commission’s accreditation. At issue were cases of accredited hospitals where patients got grossly inadequate treatment. One was a 14-bed West Texas facility where a patient died and federal officials reported that numerous staff members did not have adequate training for the medical procedures they were performing.
As a response to critics, the commission has been working in recent years to beef up its reviews. So-called unannounced visits — in which hospitals are given 48 hours’ warning — began a few years ago. Surveyors now track patients in the operating room and question doctors about diagnoses. What’s more, the agency responds not only to patient concerns, but to news reports of problems, as they did with JPS.
Commission surveyors arrived unannounced at JPS in June following Star-Telegram reports describing the JPS trauma center as a war zone, operating rooms as chaotic, instruments broken, rooms dirty, linens threadbare. Patients were put at risk when doctors couldn’t locate lab results or get crucial reports from specialists.
"We take all complaints seriously," commission spokeswoman Elizabeth Zhani said.
The inspectors cited a list of deficiencies, including intrusions into patient privacy, use of outdated drugs, filth and a system that could not keep track of narcotics, hospital officials said.
Such disturbing findings don’t mean the accreditation process is flawed, Zhani said.
"Accreditation is just not a [survey] visit," she said. "A lot of people tend to focus on that. We don’t know everything else that goes on."
"We cannot be there 365 days of the year," Zhani said.
But critics say the commission will be hard-pressed to identify a hazard so serious that it will shut down a hospital. It didn’t pull the accreditation from Martin Luther King Jr./Drew Medical Center until a year after Cahill conducted her survey — and the news media highlighted patient deaths tied to staff errors.
The Joint Commission process "in itself is, to say the least, superficial," Cahill said. "It doesn’t tell you what the hospital is doing."
Survey problems
The Joint Commission’s plan of attack has always been to "peer-review" or educate hospitals about quality standards, not regulate them, Zhani said.
"Organizations who contract with the Joint Commission are voluntarily saying: 'We want you to come in and survey us and inspect us to see if we’re following your standards for patient care,’ " Zhani said. "They are making a commitment that they want to focus on quality improvement."
But even supporters say the survey process has weaknesses.
Hospitals, no matter the size, are surveyed at least every three years. And the inspectors — usually independent contractors, not commission employees — spend about three days, often in back-to-back interviews with hospital administrators. Some say that doesn’t allow sufficient time for investigation.
At times, medical records are reviewed to make sure the proper documentation is there, not that patients received the proper medication, critics say. The focus of the surveys is on process rather than outcome, said Emily Moreno, a San Antonio registered nurse, now retired, who spent 30 years doing quality assurance at hospitals.
Even at that, though, a surveyor might have found what the JPS consultant did — that up to 20 percent of all medical records were missing.
It also isn’t clear to what degree hospital officials direct surveyors to departments or whether surveyors can — or have time to — wander around and just freely observe. That could explain why troubling problems were apparently overlooked at JPS.
Because the Joint Commission doesn’t make its reports available to the public, patients can’t be assured that surveyors visited some of the less conspicuous areas of the hospital, such as the unit that houses Tarrant County Jail inmates.
An independent consultant in 2007 noted a "dungeon like feel" to the unit and said that infectious patients were housed with noninfectious ones, with the potential of exposing patients, staff and sheriff’s deputies to such diseases as tuberculosis. Staff members told the consultant that the toilets were usually dirty and smelly, garbage cans regularly overflowed. Furniture was broken, and workspaces put doctors and nurses at risk of attack.
The Star-Telegram was not able to interview commission inspectors, despite repeated attempts.
When the survey turns up deficiencies, the commission works with hospitals to help them meet standards. And the commission continues to unveil better methods of identifying patient safety risks. One is a new practice in which surveyors "trace" or follow patients in surgery and other critical services in order to look for vulnerabilities in a hospital’s management of their care.
But critics say that the Joint Commission has other interests that may trump patient safety and lead the commission to stymie efforts that would strengthen compliance and public awareness of hospital problems.
For one, it relies on hospitals for the lion’s share of its $108 million in revenue. Hospitals pay tens of thousands of dollars for their survey evaluations. What’s more, the commission sells its consulting services to help hospitals meet accreditation standards. That boosts sales and creates the potential for conflicts of interest.
"It’s basically run by the [hospital] industry," said Julia Greene, a healthcare professional in Chicago with the Service Employees International Union, which has 2 million members, including the nation’s largest healthcare union.
Among those seeking change is the American Nurses Association, which blames the U.S. Department of Health and Human Services for failing to adequately supervise the commission’s role in the accreditation of hospitals.
Pressure is also coming from some groups concerned about rising healthcare costs. Business groups and insurers want to know if they are getting effective care for their money.
The Office of the Inspector General of DHHS is also expected to add fuel to the fire to revamp the commission. By winter, it is expected to release an examination of the commission’s accreditation process. The inspector general last reviewed the nonprofit in a scathing 1999 report that said its inspections were superficial and left little time for real scrutiny of a hospital’s errant practitioners — the ones most apt to do harm to patients.
Checks and balances
The government does provide some checks and balances to the commission in the form of federal validation surveys, such as Cahill’s at Martin Luther King Jr./Drew, which closed in August 2007 because of substandard conditions.
The surveys are conducted to validate the processes of the commission — "to make sure they’re doing what they’re supposed to be doing," Cahill said.
At most, only 5 percent of the nation’s hospitals undergo the federal scrutiny each year, she said.
But they always involve a team of specialists who spend a longer period studying a hospital, relying on a set of 19 health quality "conditions" required under Medicare regulations. For the MLK Jr./Drew Medical Center survey, Cahill was teamed with pharmacists, nutritionists, nurses and physicians. The surveys can last up to five days, and surveyors can visit the hospital four or five times to check to see that the hospital has corrected problems.
"When [government inspectors] do a validation survey based on the federal regulations, they are very, very thorough and the survey process is a much better process at finding problems than the Joint Commission would ever be," Cahill said.
While inspectors will suggest ways to improve, the survey is intended to root out bad practices, Cahill said.
If a medical record is reviewed, for example, government inspectors observe the patient’s care to see if the practice matches the hospital’s policy and federal standards, she said.
"We probe it further until we are convinced that it’s not an issue or it is an issue," she said.
That’s how inspectors found the case of a meningitis patient who had mistakenly received a potent anticancer drug for four days at the Los Angeles hospital.
Patients may also get some protection from state enforcement agencies. In June, Texas health inspectors were quick to arrive at JPS after news reports drew the commission back for a closer look. State inspectors are closer to the action and can add more layers of protection, the DHHS inspector general reported in 2000.
And next year, there may be a new face or two arriving to accredit hospitals in Texas. A Houston-based accreditation company — DNV Healthcare — is drawing the interest of a number of hospitals who say the commission’s standards deviate too far from Medicare rules.
The commission may have to reckon with the idea that, over time, it could lose its virtual monopoly on accreditation.
That’s all right with the Texas Hospital Association.
Competition "is a good thing . . .," says Starr West, senior director of policy analysis for the association.
"This sort of levels the playing field. We’ve been very supportive of having competition . . . it kind of keeps everybody in line."
Changes in hospitals sought Consumer advocates and other critics are pressing the Joint Commission to strengthen public accountability of hospitals. These are some of the changes being sought.
1 Disclose more information, including life-threatening medical errors and hospital deficiencies.
A commission manual states that hospitals are "encouraged" to report sentinel events, including patient suicide, abduction of newborns, foreign objects left in patients’ bodies during surgery and other major events. But hospitals are not required to do so. When a hospital does report, the commission usually does not identify it to the public. A national snapshot of reported sentinel events on the Joint Commission Web site shows just a map of the number of incidents by state.
Releasing the names of hospitals where sentinel events occurred would be a small step in protecting the public, said Lisa McGiffert, senior policy analyst at Consumers Union in Austin, which publishes Consumer Reports. But even that wouldn’t provide the public with information it needs, she said. For example, the Centers for Disease Control and Prevention estimates that almost 100,000 people die of hospital-acquired infections each year, yet in the past 13 years, the Joint Commission has classified only 105 cases as sentinel events, she said.
"If these sentinel events weren’t so misleading to the public, it’d be laughable," McGiffert said.
"The public has this belief that someone else is watching and making sure that hospitals are safe, and they are mistaken. Nobody is watching."
2 Make survey reports available to the public.
On its Web site, the commission publishes "Quality Reports" that compare a hospital’s performance in a handful of areas to that of other hospitals. For example, John Peter Smith Hospital’s report shows that in 2007 it rated below most accredited hospitals in the country when it comes to heart attack care. ( www.qualitycheck.org/qualityreport.aspx?hcoid=9048#)
But it’s left up to hospitals to decide whether to disclose deficiencies noted by surveyors. After commission inspectors paid a surprise visit to JPS this summer, the hospital made public some findings but did not release the report and specific deficiencies.
"If the organization wants to release the report, they can release it," Joint Commission spokeswoman Elizabeth Zhani said.
3 Pay more heed to patient complaints.
Emily Moreno of San Antonio, who focused on hospital quality during her 30 years as a registered nurse, filed a complaint with the commission after she developed an infection at the site of a breast biopsy at an Oklahoma City hospital. The commission had accredited the hospital seven months earlier.
It took no action on her complaint, she said. It only told her, in response, that it gives "serious consideration" to all complaints.
Susan Sheridan, an Idaho mother of a child with cerebral palsy, did get the commission’s attention. Her son, now 13, suffered brain damage because of the failure of hospitals to test newborns for jaundice. She and other mothers of children with cerebral palsy co-founded Parents of Infants of Children with Kernicterus. Thanks to her advocacy, hospitals now test for excessive bilirubin levels in newborns.
4 Place more patient representatives on the commission board.
Sheridan would also like to see the commission open up more hospital data to the public, but doesn’t believe that will happen soon.
"Their very structure stifles that," she said. Most of its board members are doctors or health practitioners.
"What ties their hands basically is that its members and their clients are hospitals," she said.
Tuesday, August 19, 2008
Public Pressure Is Working
 7 SoCal Hospitals Fined For Medical Errors
7 SoCal Hospitals Fined For Medical ErrorsKTLA News August 18, 2008, 8:58 PM PDT
LOS ANGELES -- Seven hospitals in L.A. and Orange counties were fined today by the state for mistakes that included leaving surgical tools in patients and over-medicating a patient.
"Ensuring all Californians receive quality patient care is our top priority," said Kathleen Billingsley, deputy director of the Center for Health Care Quality with the California Department of Public Health.
Statewide, fines were levied against a total of 18 hospitals -- some of which had multiple violations -- for "incidents that caused, or were likely to cause, serious injury or death to patients."
Harbor-UCLA Medical Center in Torrance was fined $25,000 for failing to accurately label tissue specimens, which led to unnecessary surgery for one patient and delayed treatment for another, according to DPH. The hospital received a second $25,000 penalty for failing to provide screening examinations and stabilizing medical care in a timely manner for two patients, according to the state healthcare agency.
Los Angeles County-USC Medical Center was fined $25,000 for not providing adequate nursing staff for a suicide watch to meet the needs of a patient, DPH found.
Five hospitals in Orange County were fined $25,000 per violation cited by the state.
Anaheim General Hospital received two violations for failing to ensure medical devices were electronically safe and for failing to maintain the pharmacy's refrigerated temperatures.
Coastal Communities Hospital in Santa Ana was fined for over-medicating a patient, resulting in death.
At Fountain Valley Regional Hospital, doctors left a sponge in a patient following surgery.
At Hoag Memorial Hospital Presbyterian in Newport Beach, hospital staff left a surgical instrument in a patient.
At Los Alamitos Medical Center, a patient died after falling out of a wheelchair. State regulators found hospital staff failed to buckle the person into the chair.
The hospitals may appeal the penalties by requesting a hearing within 10 days of notification.
Friday, August 15, 2008
How to stop Hospital Infections
Fox11 News in Los Angeles reports on the very disturbing trend of more and more patients picking up stubborn, dangerous infections while in the hospital. Such was the case with necrotizing fasciitis Survivor Alicia Cole. Now Dr. Alfonso Torres Cook is fighting back against infections using a common-sense plan of attack, and drugs have nothing to do with it. More hospitals should follow his lead.
Wednesday, July 9, 2008
Anonymous Protection?
No hospitals named in tales of infection
Report on diseases acquired during medical procedures keeps institutions anonymous
By CATHLEEN F. CROWLEY, Staff writer First published: Wednesday, July 9, 2008
ALBANY -- Three years after a law requiring hospitals to report their infection rates to the state passed, the numbers have been released -- sort of.
The hospital-by-hospital rates for 2007 will not be fully disclosed. Instead, New Yorkers can see aggregate rates, an interim step negotiated by the hospital industry when the law was enacted in 2005.
"The promise that was made to (the hospital community) was we will do this right," said Assemblyman Richard Gottfried, chairman of the Assembly Health Committee, "to make sure it's running right before we take it public. That was an important promise and a smart promise."
The hospital-specific numbers will be revealed next year and every year thereafter. Meanwhile, the public can view the 2007 hospital-by-hospital rates on the state Department of Health's Web site, http://www.nyhealth.gov, but the names of the hospitals are masked.
According to the federal Centers for Disease Control and Prevention, there were an estimated 1.7 million health care-associated infections nationally and 99,000 deaths from those infections in 2002. Hospital-acquired infections are considered preventable with good hand-washing, equipment sterilization and proper procedures.
Capital Region hospitals refused a request from the Times Union to voluntarily release their infection rates on Tuesday, saying they will follow the state's timeline for unveiling the information.
"It allows us and all hospitals to take a look at the statewide data and compare it to make sure it's accurate and complete and we are all comparing apples to apples," said Brad Sexauer, Saratoga Hospital's vice president of strategy and marketing development.
Arthur Levin, director of the Center for Medical Consumers and a member of the state's advisory board for the reporting system, defended the anonymity granted to hospitals this year.
Levin said it will assure that the data is accurate, and next year, "hospitals will have no excuses."
According to the aggregate figures released Tuesday, New York's infection rates mirror the nation's. For every 100 people who undergo colon surgery, about six get an infection, according to the report. For people who have a coronary bypass graft, 3.6 out of every 100 get an infection. Fewer than 1 percent develop infections related to central lines, which are tubes that snake through a vein to the heart to deliver medicine and monitor heart function.
Of all reported infections, about 10 percent were caused by methicillin-resistant Staphylococcus aureus or MRSA. The rest were caused by organisms that respond more easily to antibiotics.
Betsy McCaughey, the former New York lieutenant governor who has since founded the Committee to Reduce Infection Deaths, said the report has many shortcomings -- most notably the comparison to national rates.
"The only infection rate that is acceptable is zero," McCaughey said.
McCaughey criticized the report for highlighting risk factors for infections, like gender and obesity, without exploring the most important contributors: unclean hospitals and lax procedures.
Report on diseases acquired during medical procedures keeps institutions anonymous
By CATHLEEN F. CROWLEY, Staff writer First published: Wednesday, July 9, 2008
ALBANY -- Three years after a law requiring hospitals to report their infection rates to the state passed, the numbers have been released -- sort of.
The hospital-by-hospital rates for 2007 will not be fully disclosed. Instead, New Yorkers can see aggregate rates, an interim step negotiated by the hospital industry when the law was enacted in 2005.
"The promise that was made to (the hospital community) was we will do this right," said Assemblyman Richard Gottfried, chairman of the Assembly Health Committee, "to make sure it's running right before we take it public. That was an important promise and a smart promise."
The hospital-specific numbers will be revealed next year and every year thereafter. Meanwhile, the public can view the 2007 hospital-by-hospital rates on the state Department of Health's Web site, http://www.nyhealth.gov, but the names of the hospitals are masked.
According to the federal Centers for Disease Control and Prevention, there were an estimated 1.7 million health care-associated infections nationally and 99,000 deaths from those infections in 2002. Hospital-acquired infections are considered preventable with good hand-washing, equipment sterilization and proper procedures.
Capital Region hospitals refused a request from the Times Union to voluntarily release their infection rates on Tuesday, saying they will follow the state's timeline for unveiling the information.
"It allows us and all hospitals to take a look at the statewide data and compare it to make sure it's accurate and complete and we are all comparing apples to apples," said Brad Sexauer, Saratoga Hospital's vice president of strategy and marketing development.
Arthur Levin, director of the Center for Medical Consumers and a member of the state's advisory board for the reporting system, defended the anonymity granted to hospitals this year.
Levin said it will assure that the data is accurate, and next year, "hospitals will have no excuses."
According to the aggregate figures released Tuesday, New York's infection rates mirror the nation's. For every 100 people who undergo colon surgery, about six get an infection, according to the report. For people who have a coronary bypass graft, 3.6 out of every 100 get an infection. Fewer than 1 percent develop infections related to central lines, which are tubes that snake through a vein to the heart to deliver medicine and monitor heart function.
Of all reported infections, about 10 percent were caused by methicillin-resistant Staphylococcus aureus or MRSA. The rest were caused by organisms that respond more easily to antibiotics.
Betsy McCaughey, the former New York lieutenant governor who has since founded the Committee to Reduce Infection Deaths, said the report has many shortcomings -- most notably the comparison to national rates.
"The only infection rate that is acceptable is zero," McCaughey said.
McCaughey criticized the report for highlighting risk factors for infections, like gender and obesity, without exploring the most important contributors: unclean hospitals and lax procedures.
Monday, July 7, 2008
100 Mistakes A Month
Serious patient errors at California hospitals disclosed in state filings
About 100 Californians a month are being harmed in adverse events considered preventable. A lawmaker proposes banning reimbursements to hospitals for some types of injuries.
By Jordan Rau, Los Angeles Times Staff Writer June 30, 2008
SACRAMENTO -- Last October, a technician at the children's hospital at Stanford University improperly connected a ventilator hose, accidentally pumping too little oxygen into a 9-day-old infant's lungs.
A month later, technicians at Dominican Hospital in Santa Cruz unintentionally placed a CT scan of one patient into the electronic file of another, leading physicians to remove the wrong person’s appendix.
Last March, Virginia Fahres, 76, died at Pomona Valley Hospital Medical Center in Pomona after a nurse gave her two drugs, neither of which her doctor had prescribed.
Those incidents were among 1,002 cases of serious medical harm disclosed by California hospitals between July 2007 and May of this year. The disclosures are the first under a state law that requires hospitals to inform health regulators of all substantial injuries to their patients.
Officially called “adverse events,” those accidents are also known as "never events" because they are considered preventable, and many safety experts say they should never happen. California patients are being injured at a rate of about 100 a month, according to data compiled by the state Department of Public Health.
"I think the never events are a wake-up call to everyone about the safety of California hospitals," said Beth Capell, a lobbyist for Health Access California, a consumer group.
Revelations of such errors have led lawmakers and hospital associations in at least seven states to protect patients from having to pay for the cost of care that went awry. In Sacramento, an assemblyman proposed a ban on reimbursing hospitals for the types of injuries tracked by the state. But when lobbyists for doctors and hospitals objected, he scaled it back to cover far fewer errors.
Four million people were admitted to California hospitals last year. State investigators found some errors occurred because hospitals failed to follow safeguards designed specifically to prevent harm.
Last July at UC San Diego Medical Center, a patient died after a nurse incorrectly programmed a medicine pump that then delivered more than twice the appropriate dose of a specialized blood pressure drug. Regulators found that the hospital's administration had been warned earlier by its own safety committee that "errors continue to occur" with that type of pump but had not taken sufficient corrective action, according to a state probe.
UC San Diego officials said they have since held repeat drills with staffers who treat patients with Flolan and examined every step in the process.
Dr. Angela Scioscia, the center's senior medical director, said the public reporting requirement is "a great opportunity to make rapid improvements" because hospitals can learn from one another's problems. "We don't want people to be afraid when they come into hospitals, because they are becoming safer and safer all the time," Scioscia said.
Under the 2006 disclosure law by state Sen. Elaine Alquist (D-Santa Clara), hospitals must inform state regulators of every occurrence of 28 different types of dangerous mistakes. Those include deaths during labor, medication errors, suicide attempts and sexual assaults.
The public health department has until 2015 to begin posting the information on the Internet, although officials said they hope to begin publishing it earlier. The most recent figures available cover the 10 months since July 2007. In that time, 466 patients developed bedsores so severe that the dead skin formed a crater or rotted through to the muscle or bone.
Another 145 patients had foreign objects such as surgical equipment left in their bodies. Thirty-four died while under anesthesia. In 41 surgeries, doctors performed the wrong procedure or operated on the wrong body part or on the wrong patient.
So far, the state Department of Public Health has levied $25,000 fines against 10 hospitals that reported adverse events. Officials said other investigations are still under way.
One hospital, Scripps Memorial in La Jolla, was fined twice for two errors that occurred last November with the same patient. First, as the patient was recovering from surgery, she was given a painkiller that is not supposed to be used after operations. When she went into respiratory arrest, the pharmacist provided a corrective medication at a dose 10 times too weak to be effective.
The patient survived. State investigators discovered that the hospital's pharmacists had not been properly instructed in the use of 10 medications, including the corrective drug, that the hospital stocked for emergencies.
The ventilator error at Stanford's Lucile Packard Children's Hospital occurred because a therapist had assembled the machine by following a diagram that had been drawn backward. Dr. Christy Sandborg, the hospital's chief of staff, said the medical team quickly noticed that the ventilator wasn't working correctly and stopped using it. The child recovered, she said, and the hospital has made changes to prevent future occurrences.
Overcrowded emergency rooms are another factor behind patient injuries. A 2006 study found that California had fewer emergency rooms per resident than any other state.
At Kaiser Foundation Hospital San Jose in March, staffers left a patient waiting in the emergency room for more than an hour after a test showed that his blood sugar was higher than the maximum measurable with a glucometer. The medics determined that he needed immediate care, but all 25 treatment bays were full. He passed out in the waiting room and died from heart failure.
About 100 Californians a month are being harmed in adverse events considered preventable. A lawmaker proposes banning reimbursements to hospitals for some types of injuries.
By Jordan Rau, Los Angeles Times Staff Writer June 30, 2008
SACRAMENTO -- Last October, a technician at the children's hospital at Stanford University improperly connected a ventilator hose, accidentally pumping too little oxygen into a 9-day-old infant's lungs.
A month later, technicians at Dominican Hospital in Santa Cruz unintentionally placed a CT scan of one patient into the electronic file of another, leading physicians to remove the wrong person’s appendix.
Last March, Virginia Fahres, 76, died at Pomona Valley Hospital Medical Center in Pomona after a nurse gave her two drugs, neither of which her doctor had prescribed.
Those incidents were among 1,002 cases of serious medical harm disclosed by California hospitals between July 2007 and May of this year. The disclosures are the first under a state law that requires hospitals to inform health regulators of all substantial injuries to their patients.
Officially called “adverse events,” those accidents are also known as "never events" because they are considered preventable, and many safety experts say they should never happen. California patients are being injured at a rate of about 100 a month, according to data compiled by the state Department of Public Health.
"I think the never events are a wake-up call to everyone about the safety of California hospitals," said Beth Capell, a lobbyist for Health Access California, a consumer group.
Revelations of such errors have led lawmakers and hospital associations in at least seven states to protect patients from having to pay for the cost of care that went awry. In Sacramento, an assemblyman proposed a ban on reimbursing hospitals for the types of injuries tracked by the state. But when lobbyists for doctors and hospitals objected, he scaled it back to cover far fewer errors.
Four million people were admitted to California hospitals last year. State investigators found some errors occurred because hospitals failed to follow safeguards designed specifically to prevent harm.
Last July at UC San Diego Medical Center, a patient died after a nurse incorrectly programmed a medicine pump that then delivered more than twice the appropriate dose of a specialized blood pressure drug. Regulators found that the hospital's administration had been warned earlier by its own safety committee that "errors continue to occur" with that type of pump but had not taken sufficient corrective action, according to a state probe.
UC San Diego officials said they have since held repeat drills with staffers who treat patients with Flolan and examined every step in the process.
Dr. Angela Scioscia, the center's senior medical director, said the public reporting requirement is "a great opportunity to make rapid improvements" because hospitals can learn from one another's problems. "We don't want people to be afraid when they come into hospitals, because they are becoming safer and safer all the time," Scioscia said.
Under the 2006 disclosure law by state Sen. Elaine Alquist (D-Santa Clara), hospitals must inform state regulators of every occurrence of 28 different types of dangerous mistakes. Those include deaths during labor, medication errors, suicide attempts and sexual assaults.
The public health department has until 2015 to begin posting the information on the Internet, although officials said they hope to begin publishing it earlier. The most recent figures available cover the 10 months since July 2007. In that time, 466 patients developed bedsores so severe that the dead skin formed a crater or rotted through to the muscle or bone.
Another 145 patients had foreign objects such as surgical equipment left in their bodies. Thirty-four died while under anesthesia. In 41 surgeries, doctors performed the wrong procedure or operated on the wrong body part or on the wrong patient.
So far, the state Department of Public Health has levied $25,000 fines against 10 hospitals that reported adverse events. Officials said other investigations are still under way.
One hospital, Scripps Memorial in La Jolla, was fined twice for two errors that occurred last November with the same patient. First, as the patient was recovering from surgery, she was given a painkiller that is not supposed to be used after operations. When she went into respiratory arrest, the pharmacist provided a corrective medication at a dose 10 times too weak to be effective.
The patient survived. State investigators discovered that the hospital's pharmacists had not been properly instructed in the use of 10 medications, including the corrective drug, that the hospital stocked for emergencies.
The ventilator error at Stanford's Lucile Packard Children's Hospital occurred because a therapist had assembled the machine by following a diagram that had been drawn backward. Dr. Christy Sandborg, the hospital's chief of staff, said the medical team quickly noticed that the ventilator wasn't working correctly and stopped using it. The child recovered, she said, and the hospital has made changes to prevent future occurrences.
Overcrowded emergency rooms are another factor behind patient injuries. A 2006 study found that California had fewer emergency rooms per resident than any other state.
At Kaiser Foundation Hospital San Jose in March, staffers left a patient waiting in the emergency room for more than an hour after a test showed that his blood sugar was higher than the maximum measurable with a glucometer. The medics determined that he needed immediate care, but all 25 treatment bays were full. He passed out in the waiting room and died from heart failure.
Wednesday, June 18, 2008
CA Hospital Infection Legislation
There are two good hospital infection bills that are moving in the CA legislature.
In a great effort, these bills were passed by the Senate and are currently in the Assembly Health Committee.
SB158, sponsored by Sen. Dean Florez and SB 1058 sponsored by Sen. Elaine Alquist.
The Committee has scheduled a hearing on June 24 for both bills. I have been asked to speak at the hearing for SB158 and share my Survivor Story in the hopes that it will touch a legislator’s heart to vote “Yes” and help save lives.
Prior to this hearing we are asking people with personal infection stories to visit legislators in Sacramento and ask them to support these bills. We don’t want these bills to die in this committee after getting this far!
There are two things you can do to help these bills pass:
If you live in the area, travel to Sacramento for the hearing. We would like to have as many members of the public there as possible, especially Survivors and family members of victims of Hospital acquired infections.
Another thing you can do is determine if your assembly member serves on the Health Committee and contact him or her.
We support these bills because they include:
SB1058 (Alquist): http://info.sen.ca.gov/cgi-bin/postquery?bill_number=sb_1058&sess=CUR&house=B&site=sen
Thanks for your support
Committee Members
Mervyn M. Dymally - Chair
Dem-52
(916) 319-2052
Assemblymember.dymally@assembly.ca.gov
Alan Nakanishi - Vice Chair
Rep-10
(916) 319-2010
Assemblymember.nakanishi@assembly.ca.gov
Patty Berg
Dem-1
(916) 319-2001
Assemblymember.berg@assembly.ca.gov
Wilmer Amina Carter
Dem-62
(916) 319-2062
Assemblymember.Carter@assembly.ca.gov
Hector De La Torre
Dem-50
(916) 319-2050
Assemblymember.DeLaTorre@assembly.ca.gov
Kevin de Leon
Dem-45
(916) 319-2045
Assemblymember.deLeon@assembly.ca.gov
Bill Emmerson
Rep-63
(916) 319-2063
Assemblymember.emmerson@assembly.ca.gov
Ted Gaines
Rep-4
(916) 319-2004
Assemblymember.Gaines@assembly.ca.gov
Loni Hancock
Dem-14
(916) 319-2014
Assemblymember.hancock@assembly.ca.gov
Mary Hayashi
Dem-18
(916) 319-2018
Assemblymember.Hayashi@assembly.ca.gov
Edward P. Hernandez
Dem-57
(916) 319-2057
Assemblymember.Hernandez@assembly.ca.gov
Bob Huff
Rep-60
(916) 319-2060
Assemblymember.huff@assembly.ca.gov
Dave Jones
Dem-9
(916) 319-2009
Assemblymember.jones@assembly.ca.gov
Sally J. Lieber
Dem-22
(916) 319-2022
Assemblywoman.lieber@assembly.ca.gov
Fiona Ma
Dem-12
(916) 319-2012
Assemblymember.Ma@assembly.ca.gov
Mary Salas
Dem-79
(916) 319-2079
Assemblymember.Salas@assembly.ca.gov
Audra Strickland
Rep-37
(916) 319-2037
Assemblymember.strickland@assembly.ca.gov
In a great effort, these bills were passed by the Senate and are currently in the Assembly Health Committee.
SB158, sponsored by Sen. Dean Florez and SB 1058 sponsored by Sen. Elaine Alquist.
The Committee has scheduled a hearing on June 24 for both bills. I have been asked to speak at the hearing for SB158 and share my Survivor Story in the hopes that it will touch a legislator’s heart to vote “Yes” and help save lives.
Prior to this hearing we are asking people with personal infection stories to visit legislators in Sacramento and ask them to support these bills. We don’t want these bills to die in this committee after getting this far!
There are two things you can do to help these bills pass:
If you live in the area, travel to Sacramento for the hearing. We would like to have as many members of the public there as possible, especially Survivors and family members of victims of Hospital acquired infections.
Another thing you can do is determine if your assembly member serves on the Health Committee and contact him or her.
We support these bills because they include:
- Public reporting of hospital acquired infection rates
- Screening for MRSA or other effective prevention techniques
- State oversight on hospital cleaning practices and policies
- State agency oversight on infection issues
Links to the bills:
SB158 (Florez): http://info.sen.ca.gov/cgi-bin/postquery?bill_number=sb_158&sess=CUR&house=B&site=senSB1058 (Alquist): http://info.sen.ca.gov/cgi-bin/postquery?bill_number=sb_1058&sess=CUR&house=B&site=sen
Thanks for your support
Committee Members
Mervyn M. Dymally - Chair
Dem-52
(916) 319-2052
Assemblymember.dymally@assembly.ca.gov
Alan Nakanishi - Vice Chair
Rep-10
(916) 319-2010
Assemblymember.nakanishi@assembly.ca.gov
Patty Berg
Dem-1
(916) 319-2001
Assemblymember.berg@assembly.ca.gov
Wilmer Amina Carter
Dem-62
(916) 319-2062
Assemblymember.Carter@assembly.ca.gov
Hector De La Torre
Dem-50
(916) 319-2050
Assemblymember.DeLaTorre@assembly.ca.gov
Kevin de Leon
Dem-45
(916) 319-2045
Assemblymember.deLeon@assembly.ca.gov
Bill Emmerson
Rep-63
(916) 319-2063
Assemblymember.emmerson@assembly.ca.gov
Ted Gaines
Rep-4
(916) 319-2004
Assemblymember.Gaines@assembly.ca.gov
Loni Hancock
Dem-14
(916) 319-2014
Assemblymember.hancock@assembly.ca.gov
Mary Hayashi
Dem-18
(916) 319-2018
Assemblymember.Hayashi@assembly.ca.gov
Edward P. Hernandez
Dem-57
(916) 319-2057
Assemblymember.Hernandez@assembly.ca.gov
Bob Huff
Rep-60
(916) 319-2060
Assemblymember.huff@assembly.ca.gov
Dave Jones
Dem-9
(916) 319-2009
Assemblymember.jones@assembly.ca.gov
Sally J. Lieber
Dem-22
(916) 319-2022
Assemblywoman.lieber@assembly.ca.gov
Fiona Ma
Dem-12
(916) 319-2012
Assemblymember.Ma@assembly.ca.gov
Mary Salas
Dem-79
(916) 319-2079
Assemblymember.Salas@assembly.ca.gov
Audra Strickland
Rep-37
(916) 319-2037
Assemblymember.strickland@assembly.ca.gov
Thursday, June 12, 2008
Time Out for Patient Safety
LeapforPatientSafety.org encourages you to take the opportunity to recognize National Time Out Day on June 18th to remind every member of the surgical team how critical it is to take time out for patient safety.
Please visit our website: www.leapforpatientsafety.org for more information on how to prevent medical errors.
Please visit our website: www.leapforpatientsafety.org for more information on how to prevent medical errors.
Wednesday, June 11, 2008
No Double Dipping on Wipes & Clothes
Antibacterial wipes can spread superbugs
Cloths used in hospitals may transfer bacteria to other surfaces, study finds
updated 1:13 p.m. PT, Tues., June. 3, 2008
LONDON - Disinfectant wipes routinely used in hospitals may actually spread drug-resistant bacteria rather than kill the dangerous infections, British researchers said on Tuesday.
While the wipes killed some bacteria, a study of two hospitals showed they did not get them all and could transfer the so-called superbugs to other surfaces, Gareth Williams, a microbiologist at Cardiff University, said.
The findings presented at the American Society of Microbiology's General Meeting in Boston focused on bacteria that included methicillin-resistant Staphylococcus aureus, or MRSA.
"What we have found is there is a high risk," Williams, who led the study, said by telephone. "We need to give guidance to the staff on how to use the wipes because we found there is a possibility of cross transfer."
MRSA infections can range from boils to more severe infections of the bloodstream, lungs and surgical sites. Most cases are associated with hospitals, nursing homes or other health care facilities.
The superbug can cause life-threatening and disfiguring infections and can often only be treated with expensive, intravenous antibiotics.
Experts have been saying for years that poor hospital practices spread dangerous bacteria, and yet many studies have shown that health care workers, including doctors and nurses, often fail to even wash their hands as directed.
The findings from a study of intensive care units at two Welsh hospitals suggest that even cleaning with antimicrobial wipes may not be enough depending on how staff use them.
The researchers found that many health care workers cleaned multiple surfaces near patients, such as bed rails, monitors and tables with a single wipe and risked sweeping the infections around rather than cleaning them up.
"We found that the most effective way to prevent the risk of MRSA spread in hospital wards is to ensure the wipe is used only once on one surface," Williams said.
Copyright 2008 Reuters.
Cloths used in hospitals may transfer bacteria to other surfaces, study finds
updated 1:13 p.m. PT, Tues., June. 3, 2008
LONDON - Disinfectant wipes routinely used in hospitals may actually spread drug-resistant bacteria rather than kill the dangerous infections, British researchers said on Tuesday.
While the wipes killed some bacteria, a study of two hospitals showed they did not get them all and could transfer the so-called superbugs to other surfaces, Gareth Williams, a microbiologist at Cardiff University, said.
The findings presented at the American Society of Microbiology's General Meeting in Boston focused on bacteria that included methicillin-resistant Staphylococcus aureus, or MRSA.
"What we have found is there is a high risk," Williams, who led the study, said by telephone. "We need to give guidance to the staff on how to use the wipes because we found there is a possibility of cross transfer."
MRSA infections can range from boils to more severe infections of the bloodstream, lungs and surgical sites. Most cases are associated with hospitals, nursing homes or other health care facilities.
The superbug can cause life-threatening and disfiguring infections and can often only be treated with expensive, intravenous antibiotics.
Experts have been saying for years that poor hospital practices spread dangerous bacteria, and yet many studies have shown that health care workers, including doctors and nurses, often fail to even wash their hands as directed.
The findings from a study of intensive care units at two Welsh hospitals suggest that even cleaning with antimicrobial wipes may not be enough depending on how staff use them.
The researchers found that many health care workers cleaned multiple surfaces near patients, such as bed rails, monitors and tables with a single wipe and risked sweeping the infections around rather than cleaning them up.
"We found that the most effective way to prevent the risk of MRSA spread in hospital wards is to ensure the wipe is used only once on one surface," Williams said.
Copyright 2008 Reuters.
Wednesday, June 4, 2008
How do you say "There's been a mistake."
From Academic Medicine
The Attitudes and Experiences of Trainees Regarding Disclosing Medical Errors to Patients
Original Post 05/29/2008
Andrew A. White, MD; Thomas H. Gallagher, MD; Melissa J. Krauss, MPH; Jane Garbutt, MB, ChB; Amy D. Waterman, PhD; W. Claiborne Dunagan, MD; Victoria J. Fraser, MD; Wendy Levinson, MD; Eric B. Larson, MD, MPH Author Information
Abstract and Introduction
Abstract
Purpose: To measure trainees' attitudes and experiences regarding medical error and error disclosure.
Method: In 2003, the authors carried out a cross-sectional survey of 629 medical students (320 in their second year, 309 in their fourth year), 226 interns (159 in medicine, 67 in surgery), and 283 residents (211 in medicine, 72 in surgery), a total 1,138 trainees at two U.S. academic health centers.
Results: The response rate was 78% (889/1,138).
Most trainees (74%; 652/881) agreed that medical error is among the most serious health care problems. Nearly all (99%; 875/884) agreed serious errors should be disclosed to patients, but 87% (774/889) acknowledged at least one possible barrier, including thinking that the patient would not understand the disclosure (59%; 525/889), the patient would not want to know about the error (42%; 376/889), and the patient might sue (33%; 297/889).
Personal involvement with medical errors was common among the fourth-year students (78%; 164/209) and the residents (98%; 182/185). Among residents, 45% (83/185) reported involvement in a serious error, 34% (62/183) reported experience disclosing a serious error, and 63% (115/183) had disclosed a minor error. Whereas only 33% (289/880) of trainees had received training in error disclosure, 92% (808/881) expressed interest in such training, particularly at the time of disclosure.
Conclusions: Although many trainees had disclosed errors to patients, only a minority had been formally prepared to do so. Formal disclosure curricula, coupled with supervised practice, are necessary to prepare trainees to independently disclose errors to patients by the end of their training.
Introduction
The rise of the patient-safety movement and the publication of the Institute of Medicine report To Err is Human[1] have drawn the attention of both the public and physicians to the problem of medical errors. Physicians are increasingly expected to recognize, prevent, and properly disclose medical errors. In particular, ethical standards and guidelines that have emerged from accrediting organizations[2] and professional bodies[3] reflect a movement toward greater transparency in communicating with patients about errors. Although a few schools provide formal instruction in disclosure, these skills are largely taught via the hidden curriculum and role modeling.[4,5] There is little known regarding trainees' attitudes about and experiences with medical errors or their experience in disclosing errors to patients.
Despite the fact that patients uniformly endorse the disclosure of harmful errors,[6,7] such disclosure currently seems to be uncommon.[8,9] Emerging research is shedding new light on the disconnect between expectations that errors will be disclosed to patients and current clinical practice. Recent survey data from practicing physicians highlight their support for the general concept of disclosure and the difficulty they experience actually disclosing errors to patients.[10,11] Although less is known about trainees' attitudes and experiences regarding medical errors and their disclosure, the available literature suggests that most trainees have been personally involved with errors[9,12,13] and that discussing these events with patients presents substantial challenges for residents.[14,15] In one study, 76% of housestaff reported that they had made a serious medical error that they had not disclosed to the patient or a family member.[12]
Academic health centers can enhance transparency in health care by preparing new physicians for the challenges of recognizing and disclosing errors. Like all accredited organizations, they are also required by Joint Commission regulations to ensure patients are informed about unanticipated outcomes in their care.[2] Improving disclosure and meeting these regulatory goals require understanding how trainees perceive, experience, and disclose errors. Therefore, we undertook a multicenter cross-sectional survey of trainees to explore their attitudes and experiences regarding medical error and error disclosure.
The Attitudes and Experiences of Trainees Regarding Disclosing Medical Errors to Patients
Original Post 05/29/2008
Andrew A. White, MD; Thomas H. Gallagher, MD; Melissa J. Krauss, MPH; Jane Garbutt, MB, ChB; Amy D. Waterman, PhD; W. Claiborne Dunagan, MD; Victoria J. Fraser, MD; Wendy Levinson, MD; Eric B. Larson, MD, MPH Author Information
Abstract and Introduction
Abstract
Purpose: To measure trainees' attitudes and experiences regarding medical error and error disclosure.
Method: In 2003, the authors carried out a cross-sectional survey of 629 medical students (320 in their second year, 309 in their fourth year), 226 interns (159 in medicine, 67 in surgery), and 283 residents (211 in medicine, 72 in surgery), a total 1,138 trainees at two U.S. academic health centers.
Results: The response rate was 78% (889/1,138).
Most trainees (74%; 652/881) agreed that medical error is among the most serious health care problems. Nearly all (99%; 875/884) agreed serious errors should be disclosed to patients, but 87% (774/889) acknowledged at least one possible barrier, including thinking that the patient would not understand the disclosure (59%; 525/889), the patient would not want to know about the error (42%; 376/889), and the patient might sue (33%; 297/889).
Personal involvement with medical errors was common among the fourth-year students (78%; 164/209) and the residents (98%; 182/185). Among residents, 45% (83/185) reported involvement in a serious error, 34% (62/183) reported experience disclosing a serious error, and 63% (115/183) had disclosed a minor error. Whereas only 33% (289/880) of trainees had received training in error disclosure, 92% (808/881) expressed interest in such training, particularly at the time of disclosure.
Conclusions: Although many trainees had disclosed errors to patients, only a minority had been formally prepared to do so. Formal disclosure curricula, coupled with supervised practice, are necessary to prepare trainees to independently disclose errors to patients by the end of their training.
Introduction
The rise of the patient-safety movement and the publication of the Institute of Medicine report To Err is Human[1] have drawn the attention of both the public and physicians to the problem of medical errors. Physicians are increasingly expected to recognize, prevent, and properly disclose medical errors. In particular, ethical standards and guidelines that have emerged from accrediting organizations[2] and professional bodies[3] reflect a movement toward greater transparency in communicating with patients about errors. Although a few schools provide formal instruction in disclosure, these skills are largely taught via the hidden curriculum and role modeling.[4,5] There is little known regarding trainees' attitudes about and experiences with medical errors or their experience in disclosing errors to patients.
Despite the fact that patients uniformly endorse the disclosure of harmful errors,[6,7] such disclosure currently seems to be uncommon.[8,9] Emerging research is shedding new light on the disconnect between expectations that errors will be disclosed to patients and current clinical practice. Recent survey data from practicing physicians highlight their support for the general concept of disclosure and the difficulty they experience actually disclosing errors to patients.[10,11] Although less is known about trainees' attitudes and experiences regarding medical errors and their disclosure, the available literature suggests that most trainees have been personally involved with errors[9,12,13] and that discussing these events with patients presents substantial challenges for residents.[14,15] In one study, 76% of housestaff reported that they had made a serious medical error that they had not disclosed to the patient or a family member.[12]
Academic health centers can enhance transparency in health care by preparing new physicians for the challenges of recognizing and disclosing errors. Like all accredited organizations, they are also required by Joint Commission regulations to ensure patients are informed about unanticipated outcomes in their care.[2] Improving disclosure and meeting these regulatory goals require understanding how trainees perceive, experience, and disclose errors. Therefore, we undertook a multicenter cross-sectional survey of trainees to explore their attitudes and experiences regarding medical error and error disclosure.
Friday, May 30, 2008
Google Your Medical Records
Google unveils medical records storage plan
Beth Israel, CVS part of new service
By Jeffrey Krasner
Globe Staff / May 20, 2008
Internet search giant Google Inc. yesterday rolled out its long-awaited Google Health product, which will enable users to upload and store medical records from many sources. Local healthcare companies working with Google on the project include Beth Israel Deaconess Medical Center in Boston and CVS Caremark of Woonsocket, R.I.
Google said users can enter their personal medical records on a site with individual password protection, giving them a way to view the information from any geographic location. The company said such access is especially useful if a patient becomes ill or is injured far from his or her primary care physician.
"We believe that patients should be the stewards of their own data," said Dr. John Halamka, chief information officer at Beth Israel Deaconess, in a statement.
"Our vision is that [Beth Israel] patients will be able to electronically upload their diagnosis lists, medication lists, and allergy lists in a Google Health account and share that information with healthcare providers who currently don't have access" to Beth Israel's proprietary site, Halamka said.
Many in the healthcare industry consider electronic medical records crucial to reducing the cost of providing healthcare and eliminating medical errors. But the start-up of electronic systems has been painfully slow because few physicians and hospitals can afford to make the investment. Meantime, there are no established standards that would allow data to be shared across different medical record systems.
For Google, the service is part of a plan to boost user loyalty by giving them more reasons to log on to Google sites.
"This really puts the users' records right in their hands," said Marissa Mayer, a Google vice president. "We realize this is just the beginning."
In addition to uploading patient records, patients can also search for medical information, similar to what is offered on the popular website WebMD.
Helena Foulkes, a senior vice president at CVS Caremark, said patients who use in-pharmacy clinics will be able to store the record of their visits on Google Health. That function will be offered first in Tennessee and eventually expand to 500 MinuteClinic locations, she said. The chain is planning to open dozens of such clinics in Massachusetts.
"In today's healthcare environment, information related to an individual's overall health is often fragmented, creating gaps in the availability of data and missed opportunities to coordinate care," said Foulkes in a statement.
Yesterday, Google disclosed a first round of partners in the electronic medical record service. In addition to Beth Israel and CVS Caremark, partners include the Cleveland Clinic, Longs Drug Stores, Medco, and Walgreens Pharmacy. Google will continue to sign up partners to ensure that its users have the broadest possible access to medical information, Mayer said.
Google Health also has a variety of features intended to help users manage their healthcare. They include a link to help users find doctors by location or specialization. Another feature, called a "virtual pillbox," notifies patients when they need to take medications, and it warns of possible drug interactions.
Patient advocates and privacy specialists have expressed concern that despite password protection, sensitive health records stored online could be compromised. In recent years, data breaches have become more common, especially in the retail industry.
Google's new site already faces competition. The Mountain View, Calif., firm's biggest rival, Microsoft Corp., has introduced HealthVault, a similar service that gives users control over who sees their information.
Revolution Health, a start-up backed by former AOL chairman Steve Case, is believed to be working on a service for electronic medical records.
Material from Globe wire services was used in this report. Jeffrey Krasner can be reached at krasner@globe.com.
Beth Israel, CVS part of new service
By Jeffrey Krasner
Globe Staff / May 20, 2008
Internet search giant Google Inc. yesterday rolled out its long-awaited Google Health product, which will enable users to upload and store medical records from many sources. Local healthcare companies working with Google on the project include Beth Israel Deaconess Medical Center in Boston and CVS Caremark of Woonsocket, R.I.
Google said users can enter their personal medical records on a site with individual password protection, giving them a way to view the information from any geographic location. The company said such access is especially useful if a patient becomes ill or is injured far from his or her primary care physician.
"We believe that patients should be the stewards of their own data," said Dr. John Halamka, chief information officer at Beth Israel Deaconess, in a statement.
"Our vision is that [Beth Israel] patients will be able to electronically upload their diagnosis lists, medication lists, and allergy lists in a Google Health account and share that information with healthcare providers who currently don't have access" to Beth Israel's proprietary site, Halamka said.
Many in the healthcare industry consider electronic medical records crucial to reducing the cost of providing healthcare and eliminating medical errors. But the start-up of electronic systems has been painfully slow because few physicians and hospitals can afford to make the investment. Meantime, there are no established standards that would allow data to be shared across different medical record systems.
For Google, the service is part of a plan to boost user loyalty by giving them more reasons to log on to Google sites.
"This really puts the users' records right in their hands," said Marissa Mayer, a Google vice president. "We realize this is just the beginning."
In addition to uploading patient records, patients can also search for medical information, similar to what is offered on the popular website WebMD.
Helena Foulkes, a senior vice president at CVS Caremark, said patients who use in-pharmacy clinics will be able to store the record of their visits on Google Health. That function will be offered first in Tennessee and eventually expand to 500 MinuteClinic locations, she said. The chain is planning to open dozens of such clinics in Massachusetts.
"In today's healthcare environment, information related to an individual's overall health is often fragmented, creating gaps in the availability of data and missed opportunities to coordinate care," said Foulkes in a statement.
Yesterday, Google disclosed a first round of partners in the electronic medical record service. In addition to Beth Israel and CVS Caremark, partners include the Cleveland Clinic, Longs Drug Stores, Medco, and Walgreens Pharmacy. Google will continue to sign up partners to ensure that its users have the broadest possible access to medical information, Mayer said.
Google Health also has a variety of features intended to help users manage their healthcare. They include a link to help users find doctors by location or specialization. Another feature, called a "virtual pillbox," notifies patients when they need to take medications, and it warns of possible drug interactions.
Patient advocates and privacy specialists have expressed concern that despite password protection, sensitive health records stored online could be compromised. In recent years, data breaches have become more common, especially in the retail industry.
Google's new site already faces competition. The Mountain View, Calif., firm's biggest rival, Microsoft Corp., has introduced HealthVault, a similar service that gives users control over who sees their information.
Revolution Health, a start-up backed by former AOL chairman Steve Case, is believed to be working on a service for electronic medical records.
Material from Globe wire services was used in this report. Jeffrey Krasner can be reached at krasner@globe.com.
Wednesday, May 28, 2008
A Patient's Point of View
One of our own Advocate/Survivors Doreen Mulman has had 3 short articles published on Associated Content. Please check out her articles below!
Caring for Your Aging Parent? Please Read This
published on Wed, 28 May 2008 15:10:41 EDThttp://www.associatedcontent.com/article/783843/caring_for_your_aging_parent_please.html
Cleanliness is Next to Impossible published on Mon, 23 Jul 2007 08:41:00 EDThttp://www.associatedcontent.com/article/311453/cleanliness_is_next_to_impossible.html
Necrotizing Fasciitis A Survivors Story
published on Wed, 11 Apr 2007 09:08:00 EDThttp://www.associatedcontent.com/article/190560/necrotizing_fasciitis_a_survivors_story.html
Caring for Your Aging Parent? Please Read This
published on Wed, 28 May 2008 15:10:41 EDThttp://www.associatedcontent.com/article/783843/caring_for_your_aging_parent_please.html
Cleanliness is Next to Impossible published on Mon, 23 Jul 2007 08:41:00 EDThttp://www.associatedcontent.com/article/311453/cleanliness_is_next_to_impossible.html
Necrotizing Fasciitis A Survivors Story
published on Wed, 11 Apr 2007 09:08:00 EDThttp://www.associatedcontent.com/article/190560/necrotizing_fasciitis_a_survivors_story.html
Tuesday, May 27, 2008
MRSA Survivors Network
MRSA Survivors Network launches their new web site (http://www.mrsasurvivors.org/) and blog. MSN is the first consumer organization in the U.S. to raise the alarm about MRSA and healthcare-acquired infections. MSN has been the catalyst for prevention of MRSA infections by initiating groundbreaking legislation mandating MRSA screening and reporting.
The new web site will cover the latest issues concerning MRSA and is both consumer and healthcare professional driven to change the course of this disease.
For more information contact Jeanine Thomas at: jthomas@mrsasurvivors.org
The new web site will cover the latest issues concerning MRSA and is both consumer and healthcare professional driven to change the course of this disease.
For more information contact Jeanine Thomas at: jthomas@mrsasurvivors.org
Tuesday, May 20, 2008
Doctors On The Take
http://www.twincities.com/ci_9316658?IADID=Search-www.twincities.com-www.twincities.com
St. Paul Pioneer Press
Part III in our series
Critics say drug firms' payments to doctors are conflict of interest
What they spend: A look at drug company spending in Minnesota ― on top specialties and select psychiatrists.
By Jeremy Olson and Paul Tosto Pioneer Press
Article Last Updated: 05/20/2008 07:25:43 AM CDT
Drug companies have given $88 million in gifts, grants and fees to Minnesota doctors and caregivers since 2002, according to state payment records, including $782,000 to the two University of Minnesota psychiatrists who oversaw Dan Markingson's participation in a clinical drug trial.
A lawsuit over Markingson's suicide, which happened during the drug trial, accused Dr. Stephen Olson and Dr. S. Charles Schulz, chairman of the U's psychiatry department, of coercing the schizophrenic Markingson into the study.
The lawsuit, brought by Markingson's mother, Mary Weiss, charged that the doctors were under pressure to recruit patients such as Markingson to maximize payments from AstraZeneca and gain prestige by participating in the drug company's national study.
Both doctors said in court depositions that their roles were appropriate and that the money didn't influence their decisions over Markingson ― including when his mother argued that he wasn't getting better in the study and should be withdrawn.
Schulz was dismissed from the lawsuit in February; Olson settled this spring for an amount a university official described as little more than court costs. Federal reviews of the death didn't result in any penalties against the doctors or the university.
The case nonetheless offered an inside look at the kind of financial payments to doctors that some health policy experts and congressional representatives say should be restricted or at least fully disclosed to the public.
It also scrutinized the ethics of drug company funding of research ― something that has received less public attention and criticism than the free lunches, dinners and trips that drug companies have provided to doctors to promote their drugs.
Markingson, 27, killed himself May 8, 2004, in the bathroom of a West St. Paul halfway house. He had been enrolled for more than five months in the university's "CAFE" study, which compared three antipsychotic drugs.
Weiss sued the university and the psychiatrists. In an interview, she said doctors have a conflict of interest when they are financially benefiting from studies and caring for patients in those studies at the same time.
"I think they lose sight that these are people," she said, "not their own special little guinea pigs."
Minnesota is unique in requiring drug companies to report how much money they give to each doctor, but the reporting system has limitations. It doesn't always distinguish between money for a doctor's travel expenses and money for a research trial, nor does it distinguish money that was in a doctor's name but was passed directly to a research institution.
U.S. Sen. Chuck Grassley, R-Iowa, is urging a national reporting system. Grassley held a hearing last year in which two doctors said their colleagues have become trapped by the lures and pressures of drug company money.
"Physicians face a difficult choice," testified Dr. Greg Rosenthal, an Ohio eye specialist. "One path is to go along. With drug company money, you can increase your income, prestige, build your practice or fund a department, research or professorships. The middle ground is to simply look away. The hard choice is to fight back."
Olson received $220,000 from six companies since 2002, including $149,000 from AstraZeneca, according to the state records. Schulz received $562,000, including $112,000 as a researcher and consultant to AstraZeneca.
Olson said his AstraZeneca money went straight to the U but did support his salary. Markingson's full participation in the yearlong study meant up to $15,000 for the university.
The amounts aren't unusual, according to the payment records collected by the Minnesota Board of Pharmacy. The records, which were updated this month to include 2007 figures, show 167 Minnesota doctors who have received $100,000 or more since 2002. One in four psychiatrists has received funding from pharmaceutical companies, averaging about $50,000 over the six years.
Greater awareness of drug company payments has prompted tighter rules among some Minnesota health care organizations. The Mayo Clinic prohibits its doctors from being paid by drug companies to serve on their speaker's bureaus. Doctors in speaker's bureaus give lectures to other doctors about the company's medications.
The St. Mary's clinic system in Duluth recently banned pens, mugs or other freebies bearing drug company logos.
There have been fewer steps to restrict drug company funding of research, though most medical journals long ago required doctors to disclose the funding source of any research results they publish. Some health officials are now questioning the drug companies' use of "ghostwriters" to revise articles about research results to promote the drugs they sell.
Many universities view industry-sponsored research as a necessity amid tightening state and federal science budgets. Drug company funding makes up less than 7 percent of the psychiatry department budget at the University of Minnesota, but Schulz said it is needed as the U tries to move up the list of top-funded U.S. research institutions.
Since Olson was recruited in 2001 to boost the university's expertise in schizophrenia, he has led the U's efforts in three drug trials funded by AstraZeneca. He also took part in the federally funded "CATIE" trial, which suggested that older antipsychotic drugs were as effective as AstraZeneca's Seroquel and other newer drugs.
A growing body of research suggests that drug company money has an influence on study outcomes. One analysis found that industry-funded research was four to five times more likely to produce positive outcomes for a paying company's drug than federally funded research. A report last year found that drug company-funded studies of cholesterol medications were much more likely to produce results that favored their own drugs as well.
The CAFE results didn't show that AstraZeneca's Seroquel offered much benefit over two competitors ― Zyprexa and Risperdal. Patients gained control over schizophrenic symptoms and tended to stop taking the medications at the same rate, regardless of which drug they took. The level of unhealthy weight gain was comparable, too, albeit slightly higher among the Zyprexa patients.
Weiss sued AstraZeneca as well, though the company also was dismissed from the lawsuit. Her attorneys argued that AstraZeneca's goal with the CAFE study was to gain a marketing edge and that the company used selective information from the study to promote Seroquel.
The attorneys cited internal documents, which have been sealed under court order, in which AstraZeneca discussed its use of ghostwriters and strategies to present CAFE results in a way that "sells" Seroquel.
AstraZeneca declined to discuss documents from the case, but brand corporate affairs manager Abigail Baron said the company's financial arrangements with doctors are necessary to improve health through drug discovery.
"That mission cannot be fulfilled," she said, "without close partnership with those on the front lines of patient care and ... research."
Jeremy Olson can be reached at 651-228-5583 or jolson@pioneerpress.com. Paul Tosto can be reached at 651-228-2119 or ptosto@pioneerpress.com.
How Much?
Visit our database of drug company payments to Minnesota doctors Related to This series
Patient's suicide raises questions
Dan Markingson had delusions. His mother feared that the worst would happen. Then it did.
St. Paul Pioneer Press
Part III in our series
Critics say drug firms' payments to doctors are conflict of interest
What they spend: A look at drug company spending in Minnesota ― on top specialties and select psychiatrists.
By Jeremy Olson and Paul Tosto Pioneer Press
Article Last Updated: 05/20/2008 07:25:43 AM CDT
Drug companies have given $88 million in gifts, grants and fees to Minnesota doctors and caregivers since 2002, according to state payment records, including $782,000 to the two University of Minnesota psychiatrists who oversaw Dan Markingson's participation in a clinical drug trial.
A lawsuit over Markingson's suicide, which happened during the drug trial, accused Dr. Stephen Olson and Dr. S. Charles Schulz, chairman of the U's psychiatry department, of coercing the schizophrenic Markingson into the study.
The lawsuit, brought by Markingson's mother, Mary Weiss, charged that the doctors were under pressure to recruit patients such as Markingson to maximize payments from AstraZeneca and gain prestige by participating in the drug company's national study.
Both doctors said in court depositions that their roles were appropriate and that the money didn't influence their decisions over Markingson ― including when his mother argued that he wasn't getting better in the study and should be withdrawn.
Schulz was dismissed from the lawsuit in February; Olson settled this spring for an amount a university official described as little more than court costs. Federal reviews of the death didn't result in any penalties against the doctors or the university.
The case nonetheless offered an inside look at the kind of financial payments to doctors that some health policy experts and congressional representatives say should be restricted or at least fully disclosed to the public.
It also scrutinized the ethics of drug company funding of research ― something that has received less public attention and criticism than the free lunches, dinners and trips that drug companies have provided to doctors to promote their drugs.
Markingson, 27, killed himself May 8, 2004, in the bathroom of a West St. Paul halfway house. He had been enrolled for more than five months in the university's "CAFE" study, which compared three antipsychotic drugs.
Weiss sued the university and the psychiatrists. In an interview, she said doctors have a conflict of interest when they are financially benefiting from studies and caring for patients in those studies at the same time.
"I think they lose sight that these are people," she said, "not their own special little guinea pigs."
Minnesota is unique in requiring drug companies to report how much money they give to each doctor, but the reporting system has limitations. It doesn't always distinguish between money for a doctor's travel expenses and money for a research trial, nor does it distinguish money that was in a doctor's name but was passed directly to a research institution.
U.S. Sen. Chuck Grassley, R-Iowa, is urging a national reporting system. Grassley held a hearing last year in which two doctors said their colleagues have become trapped by the lures and pressures of drug company money.
"Physicians face a difficult choice," testified Dr. Greg Rosenthal, an Ohio eye specialist. "One path is to go along. With drug company money, you can increase your income, prestige, build your practice or fund a department, research or professorships. The middle ground is to simply look away. The hard choice is to fight back."
Olson received $220,000 from six companies since 2002, including $149,000 from AstraZeneca, according to the state records. Schulz received $562,000, including $112,000 as a researcher and consultant to AstraZeneca.
Olson said his AstraZeneca money went straight to the U but did support his salary. Markingson's full participation in the yearlong study meant up to $15,000 for the university.
The amounts aren't unusual, according to the payment records collected by the Minnesota Board of Pharmacy. The records, which were updated this month to include 2007 figures, show 167 Minnesota doctors who have received $100,000 or more since 2002. One in four psychiatrists has received funding from pharmaceutical companies, averaging about $50,000 over the six years.
Greater awareness of drug company payments has prompted tighter rules among some Minnesota health care organizations. The Mayo Clinic prohibits its doctors from being paid by drug companies to serve on their speaker's bureaus. Doctors in speaker's bureaus give lectures to other doctors about the company's medications.
The St. Mary's clinic system in Duluth recently banned pens, mugs or other freebies bearing drug company logos.
There have been fewer steps to restrict drug company funding of research, though most medical journals long ago required doctors to disclose the funding source of any research results they publish. Some health officials are now questioning the drug companies' use of "ghostwriters" to revise articles about research results to promote the drugs they sell.
Many universities view industry-sponsored research as a necessity amid tightening state and federal science budgets. Drug company funding makes up less than 7 percent of the psychiatry department budget at the University of Minnesota, but Schulz said it is needed as the U tries to move up the list of top-funded U.S. research institutions.
Since Olson was recruited in 2001 to boost the university's expertise in schizophrenia, he has led the U's efforts in three drug trials funded by AstraZeneca. He also took part in the federally funded "CATIE" trial, which suggested that older antipsychotic drugs were as effective as AstraZeneca's Seroquel and other newer drugs.
A growing body of research suggests that drug company money has an influence on study outcomes. One analysis found that industry-funded research was four to five times more likely to produce positive outcomes for a paying company's drug than federally funded research. A report last year found that drug company-funded studies of cholesterol medications were much more likely to produce results that favored their own drugs as well.
The CAFE results didn't show that AstraZeneca's Seroquel offered much benefit over two competitors ― Zyprexa and Risperdal. Patients gained control over schizophrenic symptoms and tended to stop taking the medications at the same rate, regardless of which drug they took. The level of unhealthy weight gain was comparable, too, albeit slightly higher among the Zyprexa patients.
Weiss sued AstraZeneca as well, though the company also was dismissed from the lawsuit. Her attorneys argued that AstraZeneca's goal with the CAFE study was to gain a marketing edge and that the company used selective information from the study to promote Seroquel.
The attorneys cited internal documents, which have been sealed under court order, in which AstraZeneca discussed its use of ghostwriters and strategies to present CAFE results in a way that "sells" Seroquel.
AstraZeneca declined to discuss documents from the case, but brand corporate affairs manager Abigail Baron said the company's financial arrangements with doctors are necessary to improve health through drug discovery.
"That mission cannot be fulfilled," she said, "without close partnership with those on the front lines of patient care and ... research."
Jeremy Olson can be reached at 651-228-5583 or jolson@pioneerpress.com. Paul Tosto can be reached at 651-228-2119 or ptosto@pioneerpress.com.
How Much?
Visit our database of drug company payments to Minnesota doctors Related to This series
Patient's suicide raises questions
Dan Markingson had delusions. His mother feared that the worst would happen. Then it did.
Monday, May 19, 2008
Let's Set Our Standards High!
Zero Tolerance for Infections: A Winning Strategy
Kelly M. Pyrek01/24/2008
“Zero tolerance” is quickly becoming the new watchword in infection prevention, as the concept of striving for zero infiltrates U.S. hospital staffs as they strive to meet new pay-for-performance mandates from the Centers for Medicare and Medicaid Services (CMS), this fall and to address healthcare-associated infections (HAIs) as “never-events.”
How did we get here? The data tell the clearest story. Approximately 2 million healthcare-associated infections (HAIs) occur annually in U.S. healthcare facilities, lead to 60,000-90,000 deaths and cost anywhere from $17 billion to $29 billion. Five percent to 15 percent of all hospitalized patients in developing countries develop an HAI; more than three-quarters of these infections are urinary tract infections, bloodstream infections (BSIs), pneumonia or surgical site infections (SSIs).1 Not only are patients sicker, pathogens are becoming stronger in their ability to shrug off microbicides, making for a potential train wreck of epic proportions unless our course is diverted.
At no time in history have healthcare institution infection prevention and control programs been more critical than they are today, and they are being supplemented by collaboratives and initiatives from public- and private-sector groups agitating for change and a recognition that something must be done to address increasing prevalence of hospital- and community-acquired infections. The Joint Commission has long required its accredited facilities to observe its patient safety goals, including preventing infections. It has been joined in recent years by a number of other agencies hoping to curb infections, including the Institute for Healthcare Improvement (IHI) and the Surgical Care Improvement Project (SCIP), as well as consumer watchdog groups such as Consumers Union and the Committee to Reduce Infection Deaths (RID). The call for public disclosure of infection rates is sweeping the country, and the MRSA scare several months ago has capitulated the angst Americans are feeling over opportunistic infections.
Infections are now on the radar of hospital administrators thanks to the aforementioned pay-for-performance mandates issued by CMS which is clamping down Oct. 1 on hospital reimbursement for complications relating to infections. Infection control practitioners (ICPs), who have long been the front-line defenders against infections and adverse events, find themselves needing to bone up on risk management principles and fiscal concepts as they attempt to tally up the high costs of infections and make the business case for infection prevention.
In the midst of this turning tide are the other healthcare workers (HCWs) responsible for providing medical and surgical care to patients and who have been blamed as the guilty party for ignoring infection prevention principles and best practices — all in a daily rush to do their jobs amidst staffing and resource shortages triggered by razor-thin hospital budgets that keep getting thinner. Although healthcare workers know what to do, they don’t always do it.
Behavior modification and cultural change is the answer, as is a call for a transition from benchmarking to zero tolerance. But there are degrees of behavior modification initiatives, from the warm and fuzzy, to the punitive and everything in between. Denise M. Murphy, MPH, BSN, RN, CIC, president of the Association for Professionals in Infection Control and Epidemiology (APIC) and the chief patient safety and quality officer at Barnes-Jewish Hospital in St. Louis, alludes to a recent meeting of public health groups that discussed zero tolerance and concerns regarding the potential for a punitive response if hospitals set the goal at zero. “This could come from healthcare executives, or even the public, when an infection occurred despite compliance with known prevention measures and where no breakdown in safe practice was found,” Murphy says. “So we settled on language stating that we’re ‘targeting zero.’ One means of eliminating as many HAIs as possible will be zero tolerance for not adhering to infection prevention measures and broken systems that lead to harm.”2
Without a national standard or model, institutions are left to decide a course of action on their own. Either way, zero tolerance is taking on new urgency as healthcare institutions decide to take a stronger stance against the number of infections previously thought to be preventable. In a 2006 story on hospital infections, Washington Post reporter Christopher Lee quotes David B. Nash, chairman of the Department of Health Policy at Thomas Jefferson University in Philadelphia, as remarking, “The new wave of research is showing that our previous expectations around what was preventable underestimated what we could actually achieve. We can prevent more infections than we thought before. Lots of hospitals are striving to get to zero.”3
Noted infection prevention expert William Jarvis, MD, of Jarvis and Associates based in Hilton Head, S.C., alludes to the struggle over just how many infections are preventable. “There has been much debate over the years,” says Jarvis, who spent 23 years at the Centers for Disease Control and Prevention (CDC). “When I was at the CDC and I would say one-third of infections are preventable, a number of people would argue, ‘that’s way too high, you can’t do that.’ But with various collaboratives and other interventions in the last five to eight years, what we have seen is that a much higher proportion of infections is preventable, whether we are talking about surgical site infections, ventilator-associated pneumonia (VAP), central line-associated bloodstream infections, or even methicillin-resistant Staphylococcus aureus (MRSA) infections. Interventions have prevented well over 50 percent and in some cases even 80 percent and 90 percent of infections, so now if we can get clinicians to implement the evidence-based recommendations that we know work, we will be very successful at preventing many infections.”
Jarvis continues, “Will we reach zero? No, but the attitude that I think we are moving toward, is one where clinicians don’t see these infections as inevitable. There are very sick patients who need a lot of invasive devices and procedures, so they are going to get infections. We need the attitude of trying to preventing all infections, and if one occurs, investigating to see what went wrong.”
Getting to zero is the basis of the “zero tolerance” of infections movement that has arisen in the last several years, promulgated by APIC. Murphy notes, “Why is the phrase ‘zero tolerance’ getting so much hype, and why should we be shaping what zero tolerance means in terms of infection prevention? Because too many people are still dying or being harmed by HAIs. We know the numbers because we compile them, but every number is someone’s loved one. Keeping people safe is the reason we do what we do — not rates. But rates and numbers measure our success so the goal must be elimination of HAIs, the metric or target must be zero. Zero is often possible. Many APIC members and their teams have set zero as the target and achieved that goal. They are truly saving lives.”2
Murphy says at that meeting among public health groups where zero tolerance was discussed, the concept was formally defined as “a culture, a goal, an attitude, and a commitment.” Murphy adds, “Infection prevention is no longer getting to a benchmark and stopping there. Zero tolerance means we must keep going, targeting zero. John Jernigan from CDC said, ‘In public health we talk about elimination all the time, about eliminating TB and other infectious diseases. So why wouldn’t we set a theoretical goal of zero even if we can’t prevent every infection because we cannot control all risk factors?’ Zero tolerance means treating every infection as if it should never happen, but when it does, we investigate the root cause. Finally, it means holding everyone accountable for HAIs, not just the ICPs.”2
Zero tolerance is creating a new infrastructure for infection prevention that includes other effective tools such as evidence-based interventions in the form of bundles. Jarvis writes, “… no single intervention prevents any HAI; rather a ‘bundle’ approach, using a package of multiple interventions based on evidence provided by the infection control community and implemented by a multidisciplinary team is the model for successful HAI prevention.” But an increasing number of researchers are acknowledging that addressing the behavioral aspects of infection prevention compliance is essential to fighting infections.1
Aboelela and colleagues4 note that attempts to address the growing problem of HAIs and their impact on healthcare systems have historically relied on infection control policies that recommend good hygiene through Standard Precautions. But they emphasize, “In order for infection control strategies to be effective, however, HCWs’ behavior must be congruent with these policies.” Aboelela and colleagues conducted a systematic review to evaluate studies testing the effectiveness of interventions aimed at changing HCWs’ behavior in reducing HAIs. Of 33 published studies, four studies reported significant reductions in HAI or colonization rates. Behavioral interventions used in these studies included an educational program, the formation of a multi-disciplinary quality improvement team, compliance monitoring and feedback, and a mandate to sign a hand hygiene requirement statement. In all 33 studies, bundles of two to five interventions were employed, making it difficult to determine the effectiveness of individual interventions. The researchers noted, “The usefulness of ‘care bundling’ has recently been recognized and recommended by the Institute for Healthcare Improvement. Considering the multifactorial nature of the HAI problem and the logistical and ethical difficulties of applying the randomized clinical trial approach to infection control research, it may be necessary to study interventions as sets of practices.”4
These practices — and their payoffs in fighting infections — have traditionally been evaluated through benchmarking activities. The one challenge with infection prevention is that much of it has been based on benchmarking among U.S. hospitals; Jarvis says that national benchmarking can be a less-than-ideal representation of infection rates across the country because it can be skewed toward large academic or teaching hospitals. “There has been this benchmarking mentality where people would look at their infection rate and then look at the CDC’s surveillance rates within the National Nosocomial Infections Surveillance System (NNIS), without realizing that they only really account for a small minority of hospitals in that system,” Jarvis explains. “Secondly, hospitals having less than 100 beds are not even allowed in that system, even though the average U.S. hospital is less than 100 beds. That means the majority of U.S. hospitals weren’t represented in that system — it was mostly academic centers that were providing the benchmarks. Everybody would look at that and say, ‘Well, if my infection rate is at or below the median of the NNIS system then I am fine, I don’t need to do anything.’”
Jarvis explains further, “The debate goes back to the mid-1970s, when the CDC conducted its landmark SENIC Project, the study of the efficacy of nosocomial infection control programs, which was a retrospective medical record chart review, and it was the first time that infection control was documented in a valid, scientific way to be cost effective and to prevent infections.” The SENIC Project was designed with three primary objectives: to determine whether (and, if so, to what degree) the implementation of infection surveillance and control programs (ISCPs) has lowered the rate of nosocomial infection, to describe the current status of ISCPs and infection rates, and to demonstrate the relationships among characteristics of hospitals and patients, components of ISCPs, and changes in the infection rate.
“At the time, no one really had a sense of what percent of infections were preventable,” Jarvis says. “Researchers conducted chart reviews and interviews about infection control programs and then they made estimates based upon the data they collected. They looked at which hospitals had epidemiologists and which had infection control professionals, how many did they have, what did they do, and then looked at programs in hospitals with lower infection rates compared to those who had higher infection rates and then made an estimate that in general, about one-third of HAIs were preventable. So it really was more of a guesstimate.”
In a 2007 white paper1 Jarvis underscored that benchmarking is inadequate and a culture of zero tolerance is required, as is a culture of accountability and administrative support. “In order to reach the goal of zero infections, hospitals need accountability,” Jarvis says. “In many U.S. hospitals, infection control programs are not well supported by administrators because they are not revenue-generating departments. Imagine a CEO at a hospital; two people from his facility offer him an option for the future; the first is the facility’s ICP. She says, ‘We can prevent many of these infections but we need more personnel. I’d like to hire one or two additional infection control personnel.’ She’s probably talking about less than $150,000 a year in salary costs, and you can prevent five infections. The next person who comes in is the chief of cardiovascular surgery who says, ‘I’d like to build a new operating room because I can do two or three coronary artery bypass procedures at $200,000 to $500,000 a pop.’ For the CEO, the decision is kind of a ‘duh!’ as to which decision he is going to make — she is always going to build the operating room.”
Jarvis continues, “Hospital CEOs and administrators must understand the importance of infection prevention and its impact on patient safety. They must realize it’s not about lip service, it’s about taking action and spending money to get to zero. There are a number of things happening that are getting the attention of hospital administrators, including CMS mandates, public reporting and other legislation at the state level. These things are bringing infections into the open, so hospital administrators are starting to see the light.”
If administrators are to see improvements in their facilities and not lose revenue from CMS, they are going to have to resolve the aforementioned issue of behavioral modification among clinicians. There are several classical battles being waged between ICPs and clinicians regarding compliance issues. One of the most enduring examples is that of hand hygiene compliance, which notoriously hovers around 30 percent to 40 percent. Studies indicate that HCWs wash their hands just one-third to one-half as often as they should.
Whitby and colleagues5 observe, “Although HCW compliance with handwashing guidelines is a cornerstone of ideal infection control practice, the rate of such compliance has proved to be abysmal.” For years, researchers have studied various interventions to discover how to improve HCWs’ knowledge of and compliance with handwashing guidelines and then reinforcing these practices. Whitby and colleagues note that “until recently, none have engendered evidence of sustained improvement during a protracted period.”
In 2000, two studies provided hope that handwashing practice could be improved. Pittet and colleagues.6 demonstrated that handwashing compliance among nurses at the University of Geneva hospitals increased to 66 percent during a 48-month period thanks to a number of interventions likely to affect HCW behavior including the provision of an alcohol-based hand rub designed to reduce the time taken and the inconvenience associated with handwashing. Larson and colleagues.7 described a significant increase in handwashing compliance that was sustained for 14 months in a Washington, D.C. teaching hospital. Their program attempted to induce organizational cultural change toward optimal hand hygiene, with senior administrative and clinical staff overtly promoting the handwashing program. Whitby and colleagues write, “Handwashing as a practice is a globally recognized phenomenon; however, the inability to motivate HCW compliance with handwashing guidelines suggests that handwashing behavior is complex. Human behavior is the result of multiple influences from our biological characteristics, environment, education, and culture.”5
For their study, Whitby and colleagues used the Theory of Planned Behavior (TPB), explaining that with regard to handwashing, TPB is “predicated on a person’s acceptance that the immediate cause of handwashing is their antecedent intention to wash their hands. The intention to perform a given behavior is predicted directly, although to differing degrees, by three variables: attitude (a feeling that the behavior is associated with certain attributes or outcomes that may or may not be beneficial to the individual), subjective norms (a person’s perception of pressure from peers and other social groups), and perceived behavioral control (a person’s perception of the ease or difficulty in performing the behavior). These variables are predicted by the strength of the person’s beliefs about the outcomes of the behavior, normative beliefs (which are based on a person’s evaluation of the expectations of peers and other social groups), and control beliefs (which are based on a person’s perception of their ability to overcome obstacles or to enhance resources that facilitate or obstruct their undertaking of the behavior).
Whitby and colleagues’ investigations focused on elucidating and determining the origin of the behavioral determinants of handwashing in nurses in the healthcare setting. According to the researchers, handwashing was perceived by the study subjects foremost as a mechanism of self protection against harmful organisms. Handwashing behavior was also influenced by the appearance of their hands. Nurses recognized that handwashing played an integral role in the removal of microbes and the prevention of their transfer, and described the practice as unconscious and habitual, rather than as a thoughtful action associated with particular occasions.
Whitby and colleagues reported that although nurses appeared to believe that they habitually washed their hands without thinking about it, a number of factors appeared to affect the importance that they placed on handwashing in the healthcare setting, including the condition of their patients, the extent of patient contact, their assessment of the task involving a patient, and workload. They write, “Nurses believed that patients are a potential reservoir of infection because patients have little understanding of infection transmission. Nurses assessed the risk of infection due to contact with individual patients on the basis of several criteria, including the patient’s diagnosis, physical appearance, and perceived general cleanliness; visibility of the patient’s body fluids; and the patient’s age. An assessment was made in terms of the degree of ‘dirtiness’ or the lack of ‘cleanliness’ of a patient. Handwashing was not always considered to be essential for certain types of physical contact with patients. Tasks that require non-intimate touching of a patient or use of inanimate objects were less likely to be considered important motivating factors for handwashing, compared with tasks involving more-prolonged physical contact. In parallel with the nurse’s assessment of the task involving a patient, nurses judged the level of ‘dirtiness’ of the actual task. This assessment resulted in nursing staff feeling compelled to wash their hands if their hands were visibly contaminated, moist or gritty, or touched axillae, genitals or the groin. Nurses reported that, when under time constraints, they used physical and task assessments to determine the necessity of handwashing. However, nurses always felt compelled to wash hands after performing tasks they considered to be ‘dirty.’”5
Whitby and colleagues point out that attitudes toward physical contamination, such as fecal material, is consistent with a hypothesis developed by Curtis and Biran, who argue that the human emotion of “disgust” is an evolutionary protective response to environmental factors that may pose a risk of infection.8 Whitby and colleagues note, “This response may be mirrored in the way that nurses make judgments about the potential risk for infection that contact with a patient may pose. Their assessment of the need to wash hands was strongly influenced by the emotional concepts of ‘dirtiness’ and ‘cleanliness.’”5 Whitby and colleagues say their data suggest that an individual’s handwashing behavior is not a homogenous practice but falls into two broad categories. The first category, “inherent handwashing practice,” occurs when hands are visibly soiled or feel sticky or gritty and requires hand cleansing with water. The second category is “elective handwashing behavior,” which does not trigger an intrinsic response with an immediate desire to wash one’s hands; it represents to the nurse an elective opportunity for handwashing. Whitby and colleagues also indicate that because of perceived time constraints, nurses appear to act through a self-developed “hierarchy of risk” to determine when handwashing was necessary, thus ranking their opportunities for handwashing. When pressed for time, nurses assign lower priority to washing their hands than they do to other more urgent tasks.5
“A major component of zero tolerance is accountability,” Jarvis emphasizes. “In general, healthcare professionals are not taught about infection prevention in medical school or nursing school. We are not reaching them at a time when we could tell them this is critical to saving lives, so as a result many of them come out of training not thinking this is very important. Also, many clinicians think infection control is the infection control department’s job, not theirs. The fact is, infection control personnel should be the hospital’s consultants — they have the knowledge of what can and should be done to prevent infections, but they are not the people putting in IV lines or putting people on ventilators, they’re not the ones not doing hand hygiene, and that’s where accountability comes in. A number of large healthcare systems are taking a very aggressive approach against MRSA because the data show we can prevent many HAIs including MRSA. I was intrigued by a very large healthcare system whose CEO contacted the CEOs of each of the hospitals and told them in 2008 their yearend bonuses would be dependent upon how well they prevented MRSA infections. If you don’t think that’s going to make clinicians have some accountability, you’re nuts. The CEOs at each of those hospitals are going to ensure their clinicians are accountable for infections because it will impact the CEOs’ pockets.”
Jarvis continues, “Behavioral changes are key. One of the biggest challenges we have had in infection control is getting clinicians to do proper hand hygiene, and we have to admit that those of us in healthcare are absolutely atrocious at achieving behavioral change. If you look at changes in behavior, whether it’s wearing seatbelts or helmets or not smoking, we don’t change our behavior because of any kind of educational program, it’s because we have to obey the law — a law is passed and you have to wear a seatbelt or you get fined. We are very poor in our understanding of the capability to change HCW behavior. It’s why hospital CEOs and administrators must move that accountability down to unit directors. A good example of this is a surgical intensive care unit. We commonly see surgeons do a tremendous job of hand hygiene before they go into the OR; they gown and glove and mask and cap, and then they scream at anyone who violates infection control in the OR. Then they finish surgery, walk out of the OR, walk into the ICU and go from patient to patient to patient and don’t do hand hygiene. That kind of behavior must become a violation in the eyes of the unit director, who warns you one time and the second time you are out of there.
“It requires a tremendous change in our culture,” Jarvis says. “For example, there are no data to show that gowning and gloving and masking in the OR actually reduces infections — there has never been a randomized controlled trial. Yet if I went into any U.S. hospital wearing the clothes I have on right now and said I want to do surgery, there would be at least four people who would tackle me before I got to the OR — even though there are no data to suggest there would be a negative impact on patients if I did that. But it’s a cultural expectation in healthcare, and I think we have to change that culture throughout our hospitals with respect to infection prevention, where everyone is expected to do the right thing. And when they’re not, others tell them they must.”
APIC provided a startling insight late last year when it released findings from a non-scientific poll of ICPs asking them about changes made in their hospitals to better address MRSA infection rates.9 This poll came on the heels of APIC’s MRSA prevalence study, written by Jarvis. “APIC’s follow-survey showed that approximately 50 percent of the ICPs who responded to the poll said they had not done more to fight MRSA because they were not given the resources they needed by their hospital administration,” Jarvis says. “I think that will continue to be an issue and it’s only through public reporting and increasing state legislation that infection control program resourcing issues will be acknowledged. With CMS penalizing hospitals on one side and legislation on the other, the two of them are going to start squeezing, and hopefully this pressure will yield results in improved resourcing, reduced infections and the kind of healthcare all of us should expect.”
Murphy provides a 10-point plan10 for getting to zero:
1. Educate all healthcare providers about infection prevention
2. Educate hospital administration about infection prevention
3. Challenge HCWs to lead the charge against HAIs
4. Influence and educate stakeholders
5. Educate the community about infection prevention
6. Use and share meaningful infection data
7. Automate more tasks in infection prevention so more time is spent on education efforts
8. Learn how to make the business case for infection prevention
9. Develop strategic partnerships
10. Keep the patient at the center of all infection prevention efforts Murphy notes,
“What else do we need to do to get to zero? We need each other. ICPs worldwide need to persist, and together we can eliminate HAIs. We can broaden the range of what’s preventable. By partnering with patients and their families and healthcare teams, with researchers, educators, standards and law makers, industry, and innovators, we can work to establish a reliable system that prevents harm from infection. We must continue to negotiate effectively to get resources needed to prevent HAIs, and then we can ‘pay it forward’ to our patients and their families.2
APIC recently announced the launch of its “Targeting Zero” Initiative. For more details, go to: http://www.infectioncontroltoday.com/hotnews/targeting-zero-initiative-launched.html
References
1. Jarvis W. The United States approach to strategies in the battle against healthcare-associated infections, 2006: transitioning from benchmarking to zero tolerance and clinician accountability. Journal of Hospital Infection. Vol. 65. Pages 3-9.
2. Murphy DM. Go for zero then pay it forward. APIC News. Fall 2007.
3. Lee C. Studies: Hospitals Could do More to Avoid Infections. The Washington Post. Nov. 21, 2006.
4. Aboelela SW, Stone PW and Larson EL. Effectiveness of bundled behavioral interventions to control healthcare-associated infections: a systematic review of the literature. Journal of Hospital Infection. Vol. 66, No. 2. Pages 101-108 June 2007.
5. Whitby M, McLaws ML, Ross MW. Why healthcare workers don’t wash their hands: A behavioral explanation. Infection Control and Hospital Epidemiology. Vol. 27, No. 5. May 2006.
6. Pittet D, Hugonnet S, Harbarth S, et al. Effectiveness of a hospital-wide program to improve compliance with hand hygiene. Lancet 2000; 356: 1307-1312.
7. Larson EL, Early E, Cloonan P, Sugrue S, Perides M. An organizational climate intervention associated with increased handwashing and decreased nosocomial infection. Behav Med 2000; 26:14-22.
8. Curtis V, Biran A. Dirt, disgust, and disease: is hygiene in our genes? Perspect Biol Med 2001; 44:17-31.
9. APIC. Survey Finds U.S. Healthcare Facilities Not Doing Enough to Curb MRSA. Accessed at: http://www.infectioncontroltoday.com/hotnews/curbing-mrsa.html
10. Time to come clean. Hospital Management. March 2007. Accessed at: http://www.hospitalmanagement.net/features/feature977/
var loc = window.location.pathname;var nt=String(Math.random()).substr(2,10);document.write ('');
Kelly M. Pyrek01/24/2008
“Zero tolerance” is quickly becoming the new watchword in infection prevention, as the concept of striving for zero infiltrates U.S. hospital staffs as they strive to meet new pay-for-performance mandates from the Centers for Medicare and Medicaid Services (CMS), this fall and to address healthcare-associated infections (HAIs) as “never-events.”
How did we get here? The data tell the clearest story. Approximately 2 million healthcare-associated infections (HAIs) occur annually in U.S. healthcare facilities, lead to 60,000-90,000 deaths and cost anywhere from $17 billion to $29 billion. Five percent to 15 percent of all hospitalized patients in developing countries develop an HAI; more than three-quarters of these infections are urinary tract infections, bloodstream infections (BSIs), pneumonia or surgical site infections (SSIs).1 Not only are patients sicker, pathogens are becoming stronger in their ability to shrug off microbicides, making for a potential train wreck of epic proportions unless our course is diverted.
At no time in history have healthcare institution infection prevention and control programs been more critical than they are today, and they are being supplemented by collaboratives and initiatives from public- and private-sector groups agitating for change and a recognition that something must be done to address increasing prevalence of hospital- and community-acquired infections. The Joint Commission has long required its accredited facilities to observe its patient safety goals, including preventing infections. It has been joined in recent years by a number of other agencies hoping to curb infections, including the Institute for Healthcare Improvement (IHI) and the Surgical Care Improvement Project (SCIP), as well as consumer watchdog groups such as Consumers Union and the Committee to Reduce Infection Deaths (RID). The call for public disclosure of infection rates is sweeping the country, and the MRSA scare several months ago has capitulated the angst Americans are feeling over opportunistic infections.
Infections are now on the radar of hospital administrators thanks to the aforementioned pay-for-performance mandates issued by CMS which is clamping down Oct. 1 on hospital reimbursement for complications relating to infections. Infection control practitioners (ICPs), who have long been the front-line defenders against infections and adverse events, find themselves needing to bone up on risk management principles and fiscal concepts as they attempt to tally up the high costs of infections and make the business case for infection prevention.
In the midst of this turning tide are the other healthcare workers (HCWs) responsible for providing medical and surgical care to patients and who have been blamed as the guilty party for ignoring infection prevention principles and best practices — all in a daily rush to do their jobs amidst staffing and resource shortages triggered by razor-thin hospital budgets that keep getting thinner. Although healthcare workers know what to do, they don’t always do it.
Behavior modification and cultural change is the answer, as is a call for a transition from benchmarking to zero tolerance. But there are degrees of behavior modification initiatives, from the warm and fuzzy, to the punitive and everything in between. Denise M. Murphy, MPH, BSN, RN, CIC, president of the Association for Professionals in Infection Control and Epidemiology (APIC) and the chief patient safety and quality officer at Barnes-Jewish Hospital in St. Louis, alludes to a recent meeting of public health groups that discussed zero tolerance and concerns regarding the potential for a punitive response if hospitals set the goal at zero. “This could come from healthcare executives, or even the public, when an infection occurred despite compliance with known prevention measures and where no breakdown in safe practice was found,” Murphy says. “So we settled on language stating that we’re ‘targeting zero.’ One means of eliminating as many HAIs as possible will be zero tolerance for not adhering to infection prevention measures and broken systems that lead to harm.”2
Without a national standard or model, institutions are left to decide a course of action on their own. Either way, zero tolerance is taking on new urgency as healthcare institutions decide to take a stronger stance against the number of infections previously thought to be preventable. In a 2006 story on hospital infections, Washington Post reporter Christopher Lee quotes David B. Nash, chairman of the Department of Health Policy at Thomas Jefferson University in Philadelphia, as remarking, “The new wave of research is showing that our previous expectations around what was preventable underestimated what we could actually achieve. We can prevent more infections than we thought before. Lots of hospitals are striving to get to zero.”3
Noted infection prevention expert William Jarvis, MD, of Jarvis and Associates based in Hilton Head, S.C., alludes to the struggle over just how many infections are preventable. “There has been much debate over the years,” says Jarvis, who spent 23 years at the Centers for Disease Control and Prevention (CDC). “When I was at the CDC and I would say one-third of infections are preventable, a number of people would argue, ‘that’s way too high, you can’t do that.’ But with various collaboratives and other interventions in the last five to eight years, what we have seen is that a much higher proportion of infections is preventable, whether we are talking about surgical site infections, ventilator-associated pneumonia (VAP), central line-associated bloodstream infections, or even methicillin-resistant Staphylococcus aureus (MRSA) infections. Interventions have prevented well over 50 percent and in some cases even 80 percent and 90 percent of infections, so now if we can get clinicians to implement the evidence-based recommendations that we know work, we will be very successful at preventing many infections.”
Jarvis continues, “Will we reach zero? No, but the attitude that I think we are moving toward, is one where clinicians don’t see these infections as inevitable. There are very sick patients who need a lot of invasive devices and procedures, so they are going to get infections. We need the attitude of trying to preventing all infections, and if one occurs, investigating to see what went wrong.”
Getting to zero is the basis of the “zero tolerance” of infections movement that has arisen in the last several years, promulgated by APIC. Murphy notes, “Why is the phrase ‘zero tolerance’ getting so much hype, and why should we be shaping what zero tolerance means in terms of infection prevention? Because too many people are still dying or being harmed by HAIs. We know the numbers because we compile them, but every number is someone’s loved one. Keeping people safe is the reason we do what we do — not rates. But rates and numbers measure our success so the goal must be elimination of HAIs, the metric or target must be zero. Zero is often possible. Many APIC members and their teams have set zero as the target and achieved that goal. They are truly saving lives.”2
Murphy says at that meeting among public health groups where zero tolerance was discussed, the concept was formally defined as “a culture, a goal, an attitude, and a commitment.” Murphy adds, “Infection prevention is no longer getting to a benchmark and stopping there. Zero tolerance means we must keep going, targeting zero. John Jernigan from CDC said, ‘In public health we talk about elimination all the time, about eliminating TB and other infectious diseases. So why wouldn’t we set a theoretical goal of zero even if we can’t prevent every infection because we cannot control all risk factors?’ Zero tolerance means treating every infection as if it should never happen, but when it does, we investigate the root cause. Finally, it means holding everyone accountable for HAIs, not just the ICPs.”2
Zero tolerance is creating a new infrastructure for infection prevention that includes other effective tools such as evidence-based interventions in the form of bundles. Jarvis writes, “… no single intervention prevents any HAI; rather a ‘bundle’ approach, using a package of multiple interventions based on evidence provided by the infection control community and implemented by a multidisciplinary team is the model for successful HAI prevention.” But an increasing number of researchers are acknowledging that addressing the behavioral aspects of infection prevention compliance is essential to fighting infections.1
Aboelela and colleagues4 note that attempts to address the growing problem of HAIs and their impact on healthcare systems have historically relied on infection control policies that recommend good hygiene through Standard Precautions. But they emphasize, “In order for infection control strategies to be effective, however, HCWs’ behavior must be congruent with these policies.” Aboelela and colleagues conducted a systematic review to evaluate studies testing the effectiveness of interventions aimed at changing HCWs’ behavior in reducing HAIs. Of 33 published studies, four studies reported significant reductions in HAI or colonization rates. Behavioral interventions used in these studies included an educational program, the formation of a multi-disciplinary quality improvement team, compliance monitoring and feedback, and a mandate to sign a hand hygiene requirement statement. In all 33 studies, bundles of two to five interventions were employed, making it difficult to determine the effectiveness of individual interventions. The researchers noted, “The usefulness of ‘care bundling’ has recently been recognized and recommended by the Institute for Healthcare Improvement. Considering the multifactorial nature of the HAI problem and the logistical and ethical difficulties of applying the randomized clinical trial approach to infection control research, it may be necessary to study interventions as sets of practices.”4
These practices — and their payoffs in fighting infections — have traditionally been evaluated through benchmarking activities. The one challenge with infection prevention is that much of it has been based on benchmarking among U.S. hospitals; Jarvis says that national benchmarking can be a less-than-ideal representation of infection rates across the country because it can be skewed toward large academic or teaching hospitals. “There has been this benchmarking mentality where people would look at their infection rate and then look at the CDC’s surveillance rates within the National Nosocomial Infections Surveillance System (NNIS), without realizing that they only really account for a small minority of hospitals in that system,” Jarvis explains. “Secondly, hospitals having less than 100 beds are not even allowed in that system, even though the average U.S. hospital is less than 100 beds. That means the majority of U.S. hospitals weren’t represented in that system — it was mostly academic centers that were providing the benchmarks. Everybody would look at that and say, ‘Well, if my infection rate is at or below the median of the NNIS system then I am fine, I don’t need to do anything.’”
Jarvis explains further, “The debate goes back to the mid-1970s, when the CDC conducted its landmark SENIC Project, the study of the efficacy of nosocomial infection control programs, which was a retrospective medical record chart review, and it was the first time that infection control was documented in a valid, scientific way to be cost effective and to prevent infections.” The SENIC Project was designed with three primary objectives: to determine whether (and, if so, to what degree) the implementation of infection surveillance and control programs (ISCPs) has lowered the rate of nosocomial infection, to describe the current status of ISCPs and infection rates, and to demonstrate the relationships among characteristics of hospitals and patients, components of ISCPs, and changes in the infection rate.
“At the time, no one really had a sense of what percent of infections were preventable,” Jarvis says. “Researchers conducted chart reviews and interviews about infection control programs and then they made estimates based upon the data they collected. They looked at which hospitals had epidemiologists and which had infection control professionals, how many did they have, what did they do, and then looked at programs in hospitals with lower infection rates compared to those who had higher infection rates and then made an estimate that in general, about one-third of HAIs were preventable. So it really was more of a guesstimate.”
In a 2007 white paper1 Jarvis underscored that benchmarking is inadequate and a culture of zero tolerance is required, as is a culture of accountability and administrative support. “In order to reach the goal of zero infections, hospitals need accountability,” Jarvis says. “In many U.S. hospitals, infection control programs are not well supported by administrators because they are not revenue-generating departments. Imagine a CEO at a hospital; two people from his facility offer him an option for the future; the first is the facility’s ICP. She says, ‘We can prevent many of these infections but we need more personnel. I’d like to hire one or two additional infection control personnel.’ She’s probably talking about less than $150,000 a year in salary costs, and you can prevent five infections. The next person who comes in is the chief of cardiovascular surgery who says, ‘I’d like to build a new operating room because I can do two or three coronary artery bypass procedures at $200,000 to $500,000 a pop.’ For the CEO, the decision is kind of a ‘duh!’ as to which decision he is going to make — she is always going to build the operating room.”
Jarvis continues, “Hospital CEOs and administrators must understand the importance of infection prevention and its impact on patient safety. They must realize it’s not about lip service, it’s about taking action and spending money to get to zero. There are a number of things happening that are getting the attention of hospital administrators, including CMS mandates, public reporting and other legislation at the state level. These things are bringing infections into the open, so hospital administrators are starting to see the light.”
If administrators are to see improvements in their facilities and not lose revenue from CMS, they are going to have to resolve the aforementioned issue of behavioral modification among clinicians. There are several classical battles being waged between ICPs and clinicians regarding compliance issues. One of the most enduring examples is that of hand hygiene compliance, which notoriously hovers around 30 percent to 40 percent. Studies indicate that HCWs wash their hands just one-third to one-half as often as they should.
Whitby and colleagues5 observe, “Although HCW compliance with handwashing guidelines is a cornerstone of ideal infection control practice, the rate of such compliance has proved to be abysmal.” For years, researchers have studied various interventions to discover how to improve HCWs’ knowledge of and compliance with handwashing guidelines and then reinforcing these practices. Whitby and colleagues note that “until recently, none have engendered evidence of sustained improvement during a protracted period.”
In 2000, two studies provided hope that handwashing practice could be improved. Pittet and colleagues.6 demonstrated that handwashing compliance among nurses at the University of Geneva hospitals increased to 66 percent during a 48-month period thanks to a number of interventions likely to affect HCW behavior including the provision of an alcohol-based hand rub designed to reduce the time taken and the inconvenience associated with handwashing. Larson and colleagues.7 described a significant increase in handwashing compliance that was sustained for 14 months in a Washington, D.C. teaching hospital. Their program attempted to induce organizational cultural change toward optimal hand hygiene, with senior administrative and clinical staff overtly promoting the handwashing program. Whitby and colleagues write, “Handwashing as a practice is a globally recognized phenomenon; however, the inability to motivate HCW compliance with handwashing guidelines suggests that handwashing behavior is complex. Human behavior is the result of multiple influences from our biological characteristics, environment, education, and culture.”5
For their study, Whitby and colleagues used the Theory of Planned Behavior (TPB), explaining that with regard to handwashing, TPB is “predicated on a person’s acceptance that the immediate cause of handwashing is their antecedent intention to wash their hands. The intention to perform a given behavior is predicted directly, although to differing degrees, by three variables: attitude (a feeling that the behavior is associated with certain attributes or outcomes that may or may not be beneficial to the individual), subjective norms (a person’s perception of pressure from peers and other social groups), and perceived behavioral control (a person’s perception of the ease or difficulty in performing the behavior). These variables are predicted by the strength of the person’s beliefs about the outcomes of the behavior, normative beliefs (which are based on a person’s evaluation of the expectations of peers and other social groups), and control beliefs (which are based on a person’s perception of their ability to overcome obstacles or to enhance resources that facilitate or obstruct their undertaking of the behavior).
Whitby and colleagues’ investigations focused on elucidating and determining the origin of the behavioral determinants of handwashing in nurses in the healthcare setting. According to the researchers, handwashing was perceived by the study subjects foremost as a mechanism of self protection against harmful organisms. Handwashing behavior was also influenced by the appearance of their hands. Nurses recognized that handwashing played an integral role in the removal of microbes and the prevention of their transfer, and described the practice as unconscious and habitual, rather than as a thoughtful action associated with particular occasions.
Whitby and colleagues reported that although nurses appeared to believe that they habitually washed their hands without thinking about it, a number of factors appeared to affect the importance that they placed on handwashing in the healthcare setting, including the condition of their patients, the extent of patient contact, their assessment of the task involving a patient, and workload. They write, “Nurses believed that patients are a potential reservoir of infection because patients have little understanding of infection transmission. Nurses assessed the risk of infection due to contact with individual patients on the basis of several criteria, including the patient’s diagnosis, physical appearance, and perceived general cleanliness; visibility of the patient’s body fluids; and the patient’s age. An assessment was made in terms of the degree of ‘dirtiness’ or the lack of ‘cleanliness’ of a patient. Handwashing was not always considered to be essential for certain types of physical contact with patients. Tasks that require non-intimate touching of a patient or use of inanimate objects were less likely to be considered important motivating factors for handwashing, compared with tasks involving more-prolonged physical contact. In parallel with the nurse’s assessment of the task involving a patient, nurses judged the level of ‘dirtiness’ of the actual task. This assessment resulted in nursing staff feeling compelled to wash their hands if their hands were visibly contaminated, moist or gritty, or touched axillae, genitals or the groin. Nurses reported that, when under time constraints, they used physical and task assessments to determine the necessity of handwashing. However, nurses always felt compelled to wash hands after performing tasks they considered to be ‘dirty.’”5
Whitby and colleagues point out that attitudes toward physical contamination, such as fecal material, is consistent with a hypothesis developed by Curtis and Biran, who argue that the human emotion of “disgust” is an evolutionary protective response to environmental factors that may pose a risk of infection.8 Whitby and colleagues note, “This response may be mirrored in the way that nurses make judgments about the potential risk for infection that contact with a patient may pose. Their assessment of the need to wash hands was strongly influenced by the emotional concepts of ‘dirtiness’ and ‘cleanliness.’”5 Whitby and colleagues say their data suggest that an individual’s handwashing behavior is not a homogenous practice but falls into two broad categories. The first category, “inherent handwashing practice,” occurs when hands are visibly soiled or feel sticky or gritty and requires hand cleansing with water. The second category is “elective handwashing behavior,” which does not trigger an intrinsic response with an immediate desire to wash one’s hands; it represents to the nurse an elective opportunity for handwashing. Whitby and colleagues also indicate that because of perceived time constraints, nurses appear to act through a self-developed “hierarchy of risk” to determine when handwashing was necessary, thus ranking their opportunities for handwashing. When pressed for time, nurses assign lower priority to washing their hands than they do to other more urgent tasks.5
“A major component of zero tolerance is accountability,” Jarvis emphasizes. “In general, healthcare professionals are not taught about infection prevention in medical school or nursing school. We are not reaching them at a time when we could tell them this is critical to saving lives, so as a result many of them come out of training not thinking this is very important. Also, many clinicians think infection control is the infection control department’s job, not theirs. The fact is, infection control personnel should be the hospital’s consultants — they have the knowledge of what can and should be done to prevent infections, but they are not the people putting in IV lines or putting people on ventilators, they’re not the ones not doing hand hygiene, and that’s where accountability comes in. A number of large healthcare systems are taking a very aggressive approach against MRSA because the data show we can prevent many HAIs including MRSA. I was intrigued by a very large healthcare system whose CEO contacted the CEOs of each of the hospitals and told them in 2008 their yearend bonuses would be dependent upon how well they prevented MRSA infections. If you don’t think that’s going to make clinicians have some accountability, you’re nuts. The CEOs at each of those hospitals are going to ensure their clinicians are accountable for infections because it will impact the CEOs’ pockets.”
Jarvis continues, “Behavioral changes are key. One of the biggest challenges we have had in infection control is getting clinicians to do proper hand hygiene, and we have to admit that those of us in healthcare are absolutely atrocious at achieving behavioral change. If you look at changes in behavior, whether it’s wearing seatbelts or helmets or not smoking, we don’t change our behavior because of any kind of educational program, it’s because we have to obey the law — a law is passed and you have to wear a seatbelt or you get fined. We are very poor in our understanding of the capability to change HCW behavior. It’s why hospital CEOs and administrators must move that accountability down to unit directors. A good example of this is a surgical intensive care unit. We commonly see surgeons do a tremendous job of hand hygiene before they go into the OR; they gown and glove and mask and cap, and then they scream at anyone who violates infection control in the OR. Then they finish surgery, walk out of the OR, walk into the ICU and go from patient to patient to patient and don’t do hand hygiene. That kind of behavior must become a violation in the eyes of the unit director, who warns you one time and the second time you are out of there.
“It requires a tremendous change in our culture,” Jarvis says. “For example, there are no data to show that gowning and gloving and masking in the OR actually reduces infections — there has never been a randomized controlled trial. Yet if I went into any U.S. hospital wearing the clothes I have on right now and said I want to do surgery, there would be at least four people who would tackle me before I got to the OR — even though there are no data to suggest there would be a negative impact on patients if I did that. But it’s a cultural expectation in healthcare, and I think we have to change that culture throughout our hospitals with respect to infection prevention, where everyone is expected to do the right thing. And when they’re not, others tell them they must.”
APIC provided a startling insight late last year when it released findings from a non-scientific poll of ICPs asking them about changes made in their hospitals to better address MRSA infection rates.9 This poll came on the heels of APIC’s MRSA prevalence study, written by Jarvis. “APIC’s follow-survey showed that approximately 50 percent of the ICPs who responded to the poll said they had not done more to fight MRSA because they were not given the resources they needed by their hospital administration,” Jarvis says. “I think that will continue to be an issue and it’s only through public reporting and increasing state legislation that infection control program resourcing issues will be acknowledged. With CMS penalizing hospitals on one side and legislation on the other, the two of them are going to start squeezing, and hopefully this pressure will yield results in improved resourcing, reduced infections and the kind of healthcare all of us should expect.”
Murphy provides a 10-point plan10 for getting to zero:
1. Educate all healthcare providers about infection prevention
2. Educate hospital administration about infection prevention
3. Challenge HCWs to lead the charge against HAIs
4. Influence and educate stakeholders
5. Educate the community about infection prevention
6. Use and share meaningful infection data
7. Automate more tasks in infection prevention so more time is spent on education efforts
8. Learn how to make the business case for infection prevention
9. Develop strategic partnerships
10. Keep the patient at the center of all infection prevention efforts Murphy notes,
“What else do we need to do to get to zero? We need each other. ICPs worldwide need to persist, and together we can eliminate HAIs. We can broaden the range of what’s preventable. By partnering with patients and their families and healthcare teams, with researchers, educators, standards and law makers, industry, and innovators, we can work to establish a reliable system that prevents harm from infection. We must continue to negotiate effectively to get resources needed to prevent HAIs, and then we can ‘pay it forward’ to our patients and their families.2
APIC recently announced the launch of its “Targeting Zero” Initiative. For more details, go to: http://www.infectioncontroltoday.com/hotnews/targeting-zero-initiative-launched.html
References
1. Jarvis W. The United States approach to strategies in the battle against healthcare-associated infections, 2006: transitioning from benchmarking to zero tolerance and clinician accountability. Journal of Hospital Infection. Vol. 65. Pages 3-9.
2. Murphy DM. Go for zero then pay it forward. APIC News. Fall 2007.
3. Lee C. Studies: Hospitals Could do More to Avoid Infections. The Washington Post. Nov. 21, 2006.
4. Aboelela SW, Stone PW and Larson EL. Effectiveness of bundled behavioral interventions to control healthcare-associated infections: a systematic review of the literature. Journal of Hospital Infection. Vol. 66, No. 2. Pages 101-108 June 2007.
5. Whitby M, McLaws ML, Ross MW. Why healthcare workers don’t wash their hands: A behavioral explanation. Infection Control and Hospital Epidemiology. Vol. 27, No. 5. May 2006.
6. Pittet D, Hugonnet S, Harbarth S, et al. Effectiveness of a hospital-wide program to improve compliance with hand hygiene. Lancet 2000; 356: 1307-1312.
7. Larson EL, Early E, Cloonan P, Sugrue S, Perides M. An organizational climate intervention associated with increased handwashing and decreased nosocomial infection. Behav Med 2000; 26:14-22.
8. Curtis V, Biran A. Dirt, disgust, and disease: is hygiene in our genes? Perspect Biol Med 2001; 44:17-31.
9. APIC. Survey Finds U.S. Healthcare Facilities Not Doing Enough to Curb MRSA. Accessed at: http://www.infectioncontroltoday.com/hotnews/curbing-mrsa.html
10. Time to come clean. Hospital Management. March 2007. Accessed at: http://www.hospitalmanagement.net/features/feature977/
var loc = window.location.pathname;var nt=String(Math.random()).substr(2,10);document.write ('');
Subscribe to:
Posts (Atom)